
Aldol condensation reaction is a special reaction of aldehydes.
A.What is an aldol condensation reaction?
B.Write the structural formula of aldol formed from ethanol.
Answer
547.5k+ views
Hint:The reaction of aldol condensation needs substrates like aldehyde, ketones and carboxylic acid. These substrates react in the presence of alkali and form \[\alpha ,\beta - unsaturated\,compounds\] . For initiation of the reaction you need an $\alpha - hydrogen$ or we can say $(\alpha - H)$ which is acidic and removes easily in the presence of base.
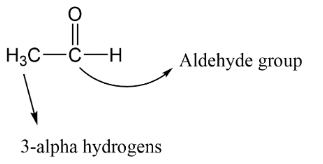
Complete step-by-step answer:A. In the aldol reaction the carbonyl compounds like aldehydes, ketone and carboxylic acid which have $\alpha - hydrogen$ in their structure reacts with the alkali and form \[\alpha ,\beta - unsaturated\,compounds\] . Now we need to take an example for better understanding, let’s say we have ethanal whose structure is shown as below having $(\alpha - H)$ .
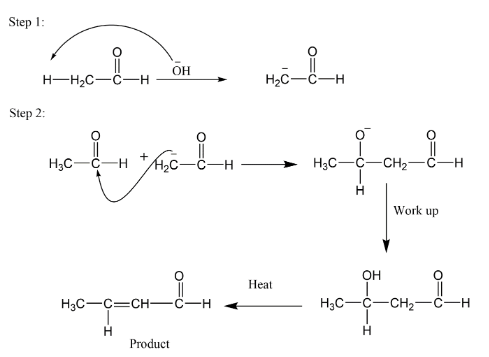
B. In case of ethanal which is an aldehyde having two carbon atoms here as we see that there are three alpha hydrogens so one hydrogen will remove in the presence of base. Let’s take sodium hydroxide $NaOH$ as base here.
So, in the presence of base only one hydrogen will remove and a carbanion will form it will act as nucleophilic reagent for another molecule of ethanal. There is a nucleophilic attack on electrophilic carbon and after work up or hydrolysis we get a hydroxyl group. If we apply heat at last, we get the product as \[\alpha ,\beta - unsaturated\,compounds\] .
Note: For finding out that whether a compound is giving the aldol reaction or not, you have to find out that it has alpha hydrogens. If substrates have alpha hydrogens then it will give the aldol reaction otherwise a famous reaction called Cannizaro reaction is performed. In that reaction we get oxidized as well as reduced product.
Complete step-by-step answer:A. In the aldol reaction the carbonyl compounds like aldehydes, ketone and carboxylic acid which have $\alpha - hydrogen$ in their structure reacts with the alkali and form \[\alpha ,\beta - unsaturated\,compounds\] . Now we need to take an example for better understanding, let’s say we have ethanal whose structure is shown as below having $(\alpha - H)$ .
B. In case of ethanal which is an aldehyde having two carbon atoms here as we see that there are three alpha hydrogens so one hydrogen will remove in the presence of base. Let’s take sodium hydroxide $NaOH$ as base here.

So, in the presence of base only one hydrogen will remove and a carbanion will form it will act as nucleophilic reagent for another molecule of ethanal. There is a nucleophilic attack on electrophilic carbon and after work up or hydrolysis we get a hydroxyl group. If we apply heat at last, we get the product as \[\alpha ,\beta - unsaturated\,compounds\] .

Note: For finding out that whether a compound is giving the aldol reaction or not, you have to find out that it has alpha hydrogens. If substrates have alpha hydrogens then it will give the aldol reaction otherwise a famous reaction called Cannizaro reaction is performed. In that reaction we get oxidized as well as reduced product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




