
Alcohols are isomeric with:
A. Acids
B. Ethers
C. Esters
D. Aldehydes
Answer
582.6k+ views
Hint:
The concept of functional isomerism along with the given class of compound can be used to deduce the isomeric class of the compound.
Complete step by step solution
We have been given chemical formulas to help us with identification or classification of the compounds and know about their properties but what if different compounds can have the same molecular formula yet different properties. We know these compounds as isomers.
Isomers can be of two types namely structural isomers and stereoisomers. Here, we will have a look at the structural isomers that can be further classified as chain isomers, position isomers, functional group isomers and metamers based on in what way the structures are different.
We are given classes of compounds with different functional groups so let’s try to understand functional group isomerism. In this case, isomers have the same molecular formula but they contain different functional groups. For example, propanone and propanal, both have the same molecular formula in spite of having ketone and aldehyde groups. We can understand this better by looking at their structures which are given below:
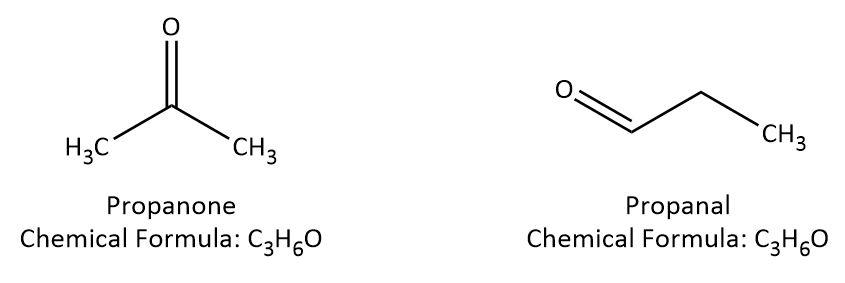
Let’s try to find out which class of compound would show functional isomerism with alcohol on the similar lines. We can take an example of alcohol, say propanol and try to see if we can have functional isomer with same molecular formula:
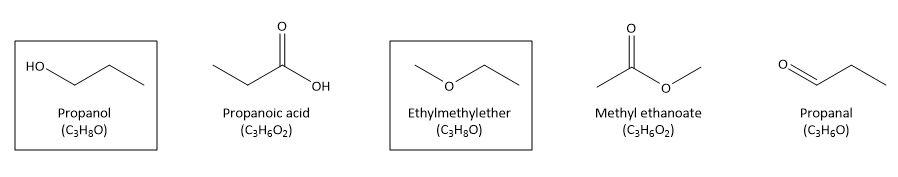
As we can see that in acid, ester and aldehyde with the same number of carbon atoms as in alcohol, the molecular formula is different. It is only in ether that the functional group is changed but the molecular formula is still the same.
Hence, the correct option is B.
Note:
We might get confused here as there in the first look it might appear that only oxygen atom has been retained in ether not the hydrogen but it will become clear by having a proper look at the structure that one hydrogen atom has been added to the terminal carbon.
The concept of functional isomerism along with the given class of compound can be used to deduce the isomeric class of the compound.
Complete step by step solution
We have been given chemical formulas to help us with identification or classification of the compounds and know about their properties but what if different compounds can have the same molecular formula yet different properties. We know these compounds as isomers.
Isomers can be of two types namely structural isomers and stereoisomers. Here, we will have a look at the structural isomers that can be further classified as chain isomers, position isomers, functional group isomers and metamers based on in what way the structures are different.
We are given classes of compounds with different functional groups so let’s try to understand functional group isomerism. In this case, isomers have the same molecular formula but they contain different functional groups. For example, propanone and propanal, both have the same molecular formula in spite of having ketone and aldehyde groups. We can understand this better by looking at their structures which are given below:

Let’s try to find out which class of compound would show functional isomerism with alcohol on the similar lines. We can take an example of alcohol, say propanol and try to see if we can have functional isomer with same molecular formula:

As we can see that in acid, ester and aldehyde with the same number of carbon atoms as in alcohol, the molecular formula is different. It is only in ether that the functional group is changed but the molecular formula is still the same.
Hence, the correct option is B.
Note:
We might get confused here as there in the first look it might appear that only oxygen atom has been retained in ether not the hydrogen but it will become clear by having a proper look at the structure that one hydrogen atom has been added to the terminal carbon.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




