
Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Answer
566.7k+ views
Hint: We have to remember that the organic compounds which comprises a hydroxyl group that is covalently linked to a carbon atom $R - OH$. Alkanes are similar to alcohols i.e. they contain long or short chains of carbon atoms surrounded by hydrogen atoms. There is less intermolecular association due to the non-polar carbon-hydrogen bond.
Complete answer:
We need to know that the alcohols have strong intermolecular hydrogen bonding. The $OH$ group present in the alcohol allows the molecules to participate in hydrogen bonding. A larger amount of energy is needed to break the intermolecular hydrogen bonding in alcohols hence; they contain higher boiling and higher melting points when compared to hydrocarbons of comparable molar mass.
On the other hand, alkanes are insoluble in water because of their non-polar nature. The majority of their bonds are nonpolar covalent carbon-hydrogen linkages. The lack of strong intermolecular forces in alkane results in the lower solubility of hydrocarbons compared to alcohols.
For example, take the example of 2-butanol and 2-methylbutane.
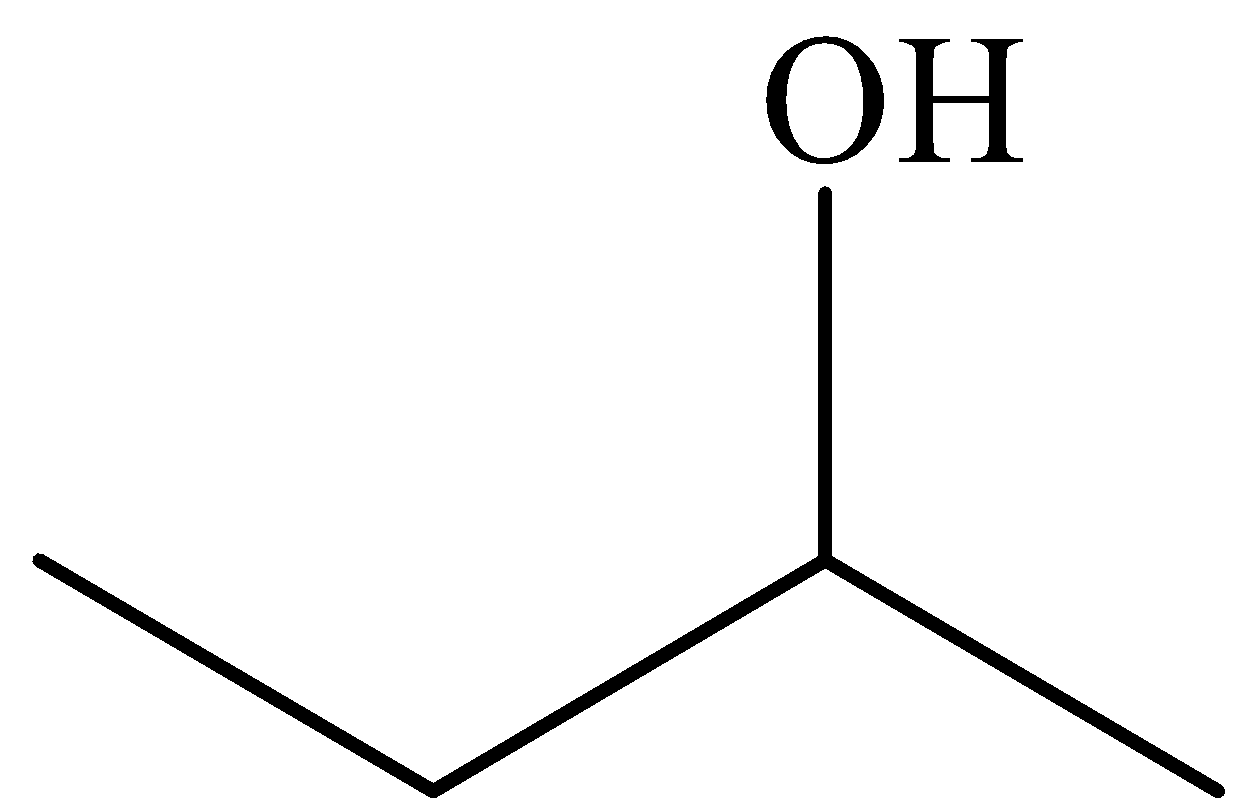
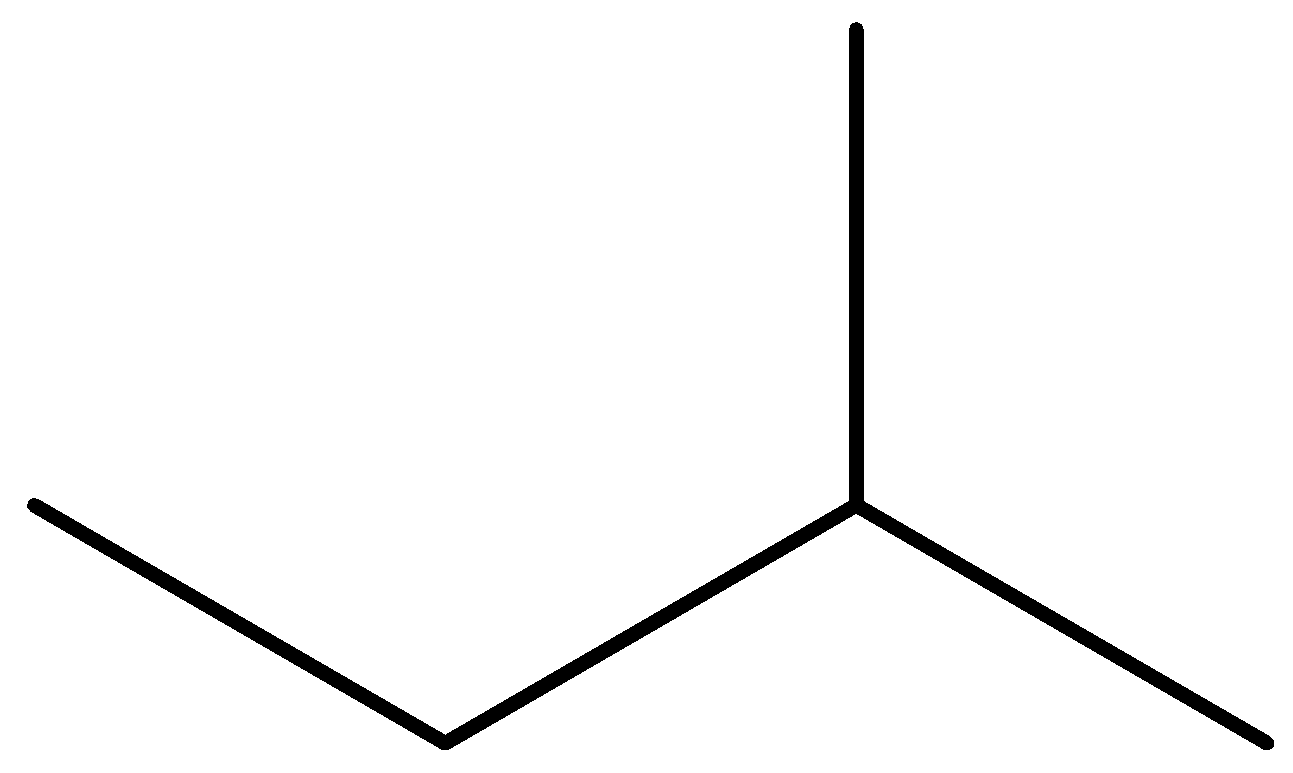
In 2-butanol, there is a strong intermolecular hydrogen bonding. The presence of hydroxyl groups permits the alcohol to take in the hydrogen bonding. A larger amount of energy is needed to break the hydrogen bonding in alcohols. Whereas, in 2-methylbutane there is no intermolecular hydrogen bonding and bonds are nonpolar covalent between carbon-hydrogen bonding. This absence of intermolecular hydrogen bonding results in the lower solubility of alkanes.
Note:
We can use alcohols in,
-As an ingredient in alcoholic beverages such as beer and wine.
-Used in the production of methylated spirit
-Used as a fuel
-Used as solvent
We can use alkanes in,
-Used for generating electricity
-Used as vehicle fuel
-Used in explosions and special effects.
Complete answer:
We need to know that the alcohols have strong intermolecular hydrogen bonding. The $OH$ group present in the alcohol allows the molecules to participate in hydrogen bonding. A larger amount of energy is needed to break the intermolecular hydrogen bonding in alcohols hence; they contain higher boiling and higher melting points when compared to hydrocarbons of comparable molar mass.
On the other hand, alkanes are insoluble in water because of their non-polar nature. The majority of their bonds are nonpolar covalent carbon-hydrogen linkages. The lack of strong intermolecular forces in alkane results in the lower solubility of hydrocarbons compared to alcohols.
For example, take the example of 2-butanol and 2-methylbutane.


In 2-butanol, there is a strong intermolecular hydrogen bonding. The presence of hydroxyl groups permits the alcohol to take in the hydrogen bonding. A larger amount of energy is needed to break the hydrogen bonding in alcohols. Whereas, in 2-methylbutane there is no intermolecular hydrogen bonding and bonds are nonpolar covalent between carbon-hydrogen bonding. This absence of intermolecular hydrogen bonding results in the lower solubility of alkanes.
Note:
We can use alcohols in,
-As an ingredient in alcoholic beverages such as beer and wine.
-Used in the production of methylated spirit
-Used as a fuel
-Used as solvent
We can use alkanes in,
-Used for generating electricity
-Used as vehicle fuel
-Used in explosions and special effects.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




