
How many alcohol isomers are possible for the formula ${ C }_{ 4 }{ H }_{ 10 }{ O }$?
a.) ${ 4 }$
b.) ${ 2 }$
c.) ${ 3 }$
d.) ${ 7 }$
Answer
593.7k+ views
Hint: The existence of two or more compounds with the same molecular formula but the different structural formula is known as isomerism; and the compounds themselves are called isomers.
Complete answer:
Geometrical isomerism: It is defined as a chemical compound having a similar molecular formula as another yet a different geometric configuration, as when atoms or groups of atoms are attached in various spatial arrangements on either side of a bond or a ring.
There are four geometrical isomers of ${ C }_{ 4 }{ H }_{ 10 }{ O }$ which are given below:
1. Butan-1-ol:
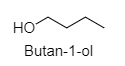
2. Butan-2-ol:
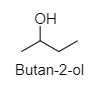
3. 2-Methyl propan-1-ol:
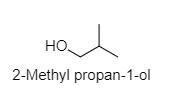
4. Methyl propan-2-ol:
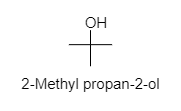
As there are four alcohol isomers of ${ C }_{ 4 }{ H }_{ 10 }{ O }$. Hence, the correct option is A.
So, the correct answer is “Option A”.
Additional Information:
Characteristics of isomers:
They have the same molecular formula but different structural formulas.
They show similar properties only when they contain the same functional group.
Two isomers can have different boiling points. For example, isomers of pentane branched-chain pentane will have less boiling point than linear because the boiling point depends on the surface area which is more in the case of n-pentane (linear).
Isomers can have different functional groups. For example, aldehyde and ketone are two isomers but they contain various functional groups.
Note: The possibility to make a mistake is that there are seven isomers of ${ C }_{ 4 }{ H }_{ 10 }{ O }$ in which four are alcoholic and 3 are ether isomers. Here, in this question alcohol isomers are asked so in spite of seven, there are four alcohol isomers.
Complete answer:
Geometrical isomerism: It is defined as a chemical compound having a similar molecular formula as another yet a different geometric configuration, as when atoms or groups of atoms are attached in various spatial arrangements on either side of a bond or a ring.
There are four geometrical isomers of ${ C }_{ 4 }{ H }_{ 10 }{ O }$ which are given below:
1. Butan-1-ol:

2. Butan-2-ol:

3. 2-Methyl propan-1-ol:

4. Methyl propan-2-ol:

As there are four alcohol isomers of ${ C }_{ 4 }{ H }_{ 10 }{ O }$. Hence, the correct option is A.
So, the correct answer is “Option A”.
Additional Information:
Characteristics of isomers:
They have the same molecular formula but different structural formulas.
They show similar properties only when they contain the same functional group.
Two isomers can have different boiling points. For example, isomers of pentane branched-chain pentane will have less boiling point than linear because the boiling point depends on the surface area which is more in the case of n-pentane (linear).
Isomers can have different functional groups. For example, aldehyde and ketone are two isomers but they contain various functional groups.
Note: The possibility to make a mistake is that there are seven isomers of ${ C }_{ 4 }{ H }_{ 10 }{ O }$ in which four are alcoholic and 3 are ether isomers. Here, in this question alcohol isomers are asked so in spite of seven, there are four alcohol isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




