
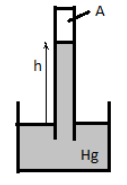
Air is trapped in the space (labeled A) above the mercury level in the tube of a barometer, which measures \[745\,{\text{mm Hg}}\]. If the atmospheric pressure is \[760\,{\text{mm Hg}}\], then the pressure of the air trapped is
A. \[15\,{\text{mm Hg}}\]
B. \[760\,{\text{mm Hg}}\]
C. Between 745 and \[760\,{\text{mm Hg}}\]
D. \[745\,{\text{mm Hg}}\]

Answer
573.9k+ views
Hint: Use the concept of pressure measurement by a barometer using the height of the mercury level in the tube of the barometer. When one tries to measure the pressure of a liquid in a beaker, the pressure exerted by the atmosphere on the liquid is equal to the sum of the pressure exerted by the mercury on the liquid and the air trapped in the tube of the barometer.
Complete step by step solution:
The mercury level in the barometer shows that pressure \[745\,{\text{mm Hg}}\]. Air is trapped above the mercury level in the tube of the barometer and the atmospheric pressure is \[760\,{\text{mm Hg}}\].
\[{P_{atm}} = 760\,{\text{mm Hg}}\]
\[P = 745\,{\text{mm Hg}}\]
The pressure exerted \[{P_{atm}}\] by the atmosphere on the mercury in the beaker and the pressure \[{P_{air}}\] exerted by the air trapped in the tube of the barometer and pressure \[P\]exerted by the mercury in the tube A must be equal.
\[{P_{atm}} = {P_{air}} + P\]
Rearrange the above equation for the pressure \[{P_{air}}\] of the air trapped in the tube of the barometer.
\[{P_{air}} = {P_{atm}} - P\]
Substitute \[760\,{\text{mm Hg}}\] for \[{P_{atm}}\]and \[745\,{\text{mm Hg}}\] for \[P\] in the above equation.
\[{P_{air}} = \left( {760\,{\text{mm Hg}}} \right) - \left( {745\,{\text{mm Hg}}} \right)\]
\[ \Rightarrow {P_{air}} = 15\,{\text{mm Hg}}\]
Therefore, the pressure of the air trapped in the tube of the barometer is \[15\,{\text{mm Hg}}\].
Hence, the correct option is A.
Additional information: The barometer measures the air pressure by balancing the weight of the air particles on the tube of the barometer and the weight of the mercury in the barometer.
If the weight of the air particles above the tube of barometer is more than the weight of the mercury then the level of the mercury drops and if the weight of the air particles above the tube of barometer is less than the weight of the mercury then the level of the mercury rises in the tube.
Note: The two pressures i.e. the atmospheric pressure and the sum of the pressure due to mercury in the barometer tube and pressure due to the air trapped in the tube must be equal otherwise the height of the mercury level in the barometer tube would not be steady.
Complete step by step solution:
The mercury level in the barometer shows that pressure \[745\,{\text{mm Hg}}\]. Air is trapped above the mercury level in the tube of the barometer and the atmospheric pressure is \[760\,{\text{mm Hg}}\].
\[{P_{atm}} = 760\,{\text{mm Hg}}\]
\[P = 745\,{\text{mm Hg}}\]
The pressure exerted \[{P_{atm}}\] by the atmosphere on the mercury in the beaker and the pressure \[{P_{air}}\] exerted by the air trapped in the tube of the barometer and pressure \[P\]exerted by the mercury in the tube A must be equal.
\[{P_{atm}} = {P_{air}} + P\]
Rearrange the above equation for the pressure \[{P_{air}}\] of the air trapped in the tube of the barometer.
\[{P_{air}} = {P_{atm}} - P\]
Substitute \[760\,{\text{mm Hg}}\] for \[{P_{atm}}\]and \[745\,{\text{mm Hg}}\] for \[P\] in the above equation.
\[{P_{air}} = \left( {760\,{\text{mm Hg}}} \right) - \left( {745\,{\text{mm Hg}}} \right)\]
\[ \Rightarrow {P_{air}} = 15\,{\text{mm Hg}}\]
Therefore, the pressure of the air trapped in the tube of the barometer is \[15\,{\text{mm Hg}}\].
Hence, the correct option is A.
Additional information: The barometer measures the air pressure by balancing the weight of the air particles on the tube of the barometer and the weight of the mercury in the barometer.
If the weight of the air particles above the tube of barometer is more than the weight of the mercury then the level of the mercury drops and if the weight of the air particles above the tube of barometer is less than the weight of the mercury then the level of the mercury rises in the tube.
Note: The two pressures i.e. the atmospheric pressure and the sum of the pressure due to mercury in the barometer tube and pressure due to the air trapped in the tube must be equal otherwise the height of the mercury level in the barometer tube would not be steady.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a labelled diagram of the human heart and label class 11 biology CBSE

What is 1s 2s 2p 3s 3p class 11 chemistry CBSE




