
What is addition polymer? Give examples.
What is PHBV? How is it useful?
Answer
580.8k+ views
Hint: Polymers are high molecular mass substances where every molecule is derived from a large number of simple molecules joined together. They are formed from repeated combinations of simplest units known as monomers and the process of formation of polymers is called polymerization.
Complete step by step answer:
(a)Addition Polymer-
An addition polymer is a polymer formed by simply linking the monomers with no co-generation of other products.
These polymers are generally made from molecules having carbon-carbon double bonds which means addition polymers are prepared from alkenes.
They are also called chain growth polymers.
Generally, these reactions occur in presence of catalyst. Main catalyst used in the polymer industry is the Ziegler Natta catalyst.
Ziegler Natta catalyst is the mixture of triethylaluminium and ${\text{TiC}}{{\text{l}}_{\text{4}}}$ .
Examples- Polyethylene, Polyvinyl Chloride, Teflon, Rubbers (Buna-N, Buna-S), Polyacrylonitrile.
One reaction of addition polymerization is given below -
$n{\text{ }}\mathop {{\text{C}}{{\text{F}}_2} = {\text{C}}{{\text{F}}_2}}\limits_{{\text{Tetrafluoroethylene}}} \xrightarrow{{{\text{Polymerisation}}}}\mathop {{{\left[ { - {\text{C}}{{\text{F}}_2} - {\text{C}}{{\text{F}}_2} - } \right]}_n}}\limits_{{\text{Teflon}}} $
In this reaction, n molecules of tetrafluoroethylene are polymerized to form Teflon. Teflon is used for non-stick coating for cooking utensils.
(b)PHBV-
PHBV is short form of ${\text{Poly }}\beta {\text{ - hydroxybutyrate - co - }}\beta {\text{ - hydroxy valerate}}$. It is obtained by copolymerization of $3 - {\text{hydroxybutanoic acid}}$ and $3 - {\text{hydroxypentanoic acid}}$. It is a biodegradable polymer. The preparation reaction is given as-
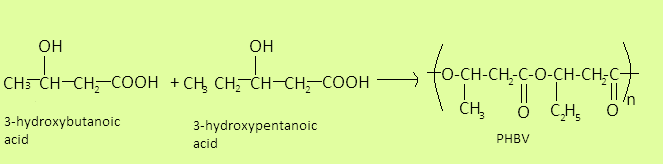
It has ester linkage (-COO) in its formula.
Uses of PHBV-
-It is used in specialty packaging.
-It is used in orthopedic devices.
-It is used in controlled release of drugs.
-It undergoes bacterial degradation in the environment hence it prevents environmental pollution.
Note:
Addition polymers are prepared in three stages-
Initiation- The substance splits into two identical parts each with one unpaired electron called free radical. The free radical then initiates the reaction by forming a bond with one of the carbon atoms having double bond.
Propagation-The new free radicals react with another monomer to add two more carbon atoms. This process is repeated.
Termination- The reaction stops when any two free radicals combine forming a covalent bond that links two chains together.
Complete step by step answer:
(a)Addition Polymer-
An addition polymer is a polymer formed by simply linking the monomers with no co-generation of other products.
These polymers are generally made from molecules having carbon-carbon double bonds which means addition polymers are prepared from alkenes.
They are also called chain growth polymers.
Generally, these reactions occur in presence of catalyst. Main catalyst used in the polymer industry is the Ziegler Natta catalyst.
Ziegler Natta catalyst is the mixture of triethylaluminium and ${\text{TiC}}{{\text{l}}_{\text{4}}}$ .
Examples- Polyethylene, Polyvinyl Chloride, Teflon, Rubbers (Buna-N, Buna-S), Polyacrylonitrile.
One reaction of addition polymerization is given below -
$n{\text{ }}\mathop {{\text{C}}{{\text{F}}_2} = {\text{C}}{{\text{F}}_2}}\limits_{{\text{Tetrafluoroethylene}}} \xrightarrow{{{\text{Polymerisation}}}}\mathop {{{\left[ { - {\text{C}}{{\text{F}}_2} - {\text{C}}{{\text{F}}_2} - } \right]}_n}}\limits_{{\text{Teflon}}} $
In this reaction, n molecules of tetrafluoroethylene are polymerized to form Teflon. Teflon is used for non-stick coating for cooking utensils.
(b)PHBV-
PHBV is short form of ${\text{Poly }}\beta {\text{ - hydroxybutyrate - co - }}\beta {\text{ - hydroxy valerate}}$. It is obtained by copolymerization of $3 - {\text{hydroxybutanoic acid}}$ and $3 - {\text{hydroxypentanoic acid}}$. It is a biodegradable polymer. The preparation reaction is given as-
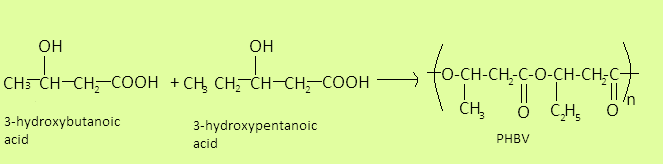
It has ester linkage (-COO) in its formula.
Uses of PHBV-
-It is used in specialty packaging.
-It is used in orthopedic devices.
-It is used in controlled release of drugs.
-It undergoes bacterial degradation in the environment hence it prevents environmental pollution.
Note:
Addition polymers are prepared in three stages-
Initiation- The substance splits into two identical parts each with one unpaired electron called free radical. The free radical then initiates the reaction by forming a bond with one of the carbon atoms having double bond.
Propagation-The new free radicals react with another monomer to add two more carbon atoms. This process is repeated.
Termination- The reaction stops when any two free radicals combine forming a covalent bond that links two chains together.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




