
What is the action of the nitrating mixture on phenol?
While separating o- and p- nitrophenol by steam distillation, Name the isomer which is steam volatile. Give reason.
Answer
547.8k+ views
Hint:Phenol is a colourless or white coloured aromatic organic compound having the molecular formula of ${C_6}{H_5}OH$. The phenol molecule comprises a phenyl group that is bonded to a hydroxyl group. For introducing nitro groups on phenol we use a nitrating mixture which is sulfuric acid and nitric acid in $1:1$ ratio. It is utilised in the nitration of organic compounds. Among the two isomers, one is having strong hydrogen bonding thus it will not easily be separable.
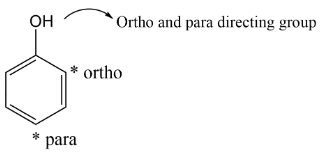
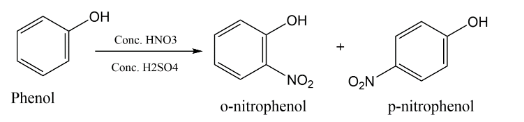
Complete step-by-step answer:When nitrating mixture reacts with Phenol, it produces the isomers of phenol i.e. p-nitrophenol and o-nitrophenol. There is an introduction of the nitro group at ortho position and para position. We get the ortho and para product because $( - OH)\,group\,is\,\left( {ortho - \,} \right)and\,\left( {para - } \right)\,directing\,group$the structures of p-nitrophenol and o-nitrophenol are displayed below:
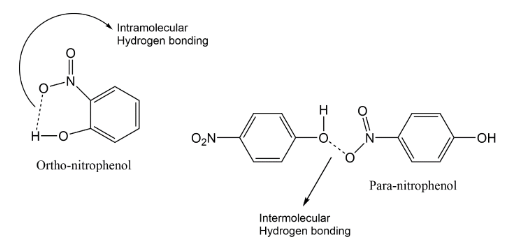
It was observed that para isomers have a high boiling point because of its symmetric structure. Thus in steam distillation due to the large difference between the boiling point it is used to separate them. Also, if we see the nitro phenols, there is intermolecular hydrogen bonding seen in para isomer.
Hydrogen bonding is an electrostatic attraction that occurs between an electronegative element and H-atom that is connected with an electronegative element. In the present case, intramolecular H-bonding (within a molecule) exists in o-nitrophenol due to which it possesses a low boiling point. While in the case of p-nitrophenol, the molecules are strongly linked owing to the existence of intermolecular H-bonding (within molecules). Therefore, o-nitrophenol is steam volatile.
Thus we get two types of isomers on nitration ortho and para, which can be separated by steam distillation. Ortho-nitrophenol is steam volatile.
Note: Steam distillation is a process which is generally employed to purify liquids that are actually immiscible with water. The liquid starts boiling when its vapour pressure becomes equal to$1\,atm$. Once the steam is made to bubble through the liquid, the mixture starts boiling when the total vapour pressure of liquid and the water becomes equal to $1\,atm$. Now, the mixture will be collected as distillate and finally, the water gets separated. Thus, the liquid can effectively be distilled at a temperature lower than its boiling point.
Complete step-by-step answer:When nitrating mixture reacts with Phenol, it produces the isomers of phenol i.e. p-nitrophenol and o-nitrophenol. There is an introduction of the nitro group at ortho position and para position. We get the ortho and para product because $( - OH)\,group\,is\,\left( {ortho - \,} \right)and\,\left( {para - } \right)\,directing\,group$the structures of p-nitrophenol and o-nitrophenol are displayed below:

It was observed that para isomers have a high boiling point because of its symmetric structure. Thus in steam distillation due to the large difference between the boiling point it is used to separate them. Also, if we see the nitro phenols, there is intermolecular hydrogen bonding seen in para isomer.
Hydrogen bonding is an electrostatic attraction that occurs between an electronegative element and H-atom that is connected with an electronegative element. In the present case, intramolecular H-bonding (within a molecule) exists in o-nitrophenol due to which it possesses a low boiling point. While in the case of p-nitrophenol, the molecules are strongly linked owing to the existence of intermolecular H-bonding (within molecules). Therefore, o-nitrophenol is steam volatile.


Thus we get two types of isomers on nitration ortho and para, which can be separated by steam distillation. Ortho-nitrophenol is steam volatile.
Note: Steam distillation is a process which is generally employed to purify liquids that are actually immiscible with water. The liquid starts boiling when its vapour pressure becomes equal to$1\,atm$. Once the steam is made to bubble through the liquid, the mixture starts boiling when the total vapour pressure of liquid and the water becomes equal to $1\,atm$. Now, the mixture will be collected as distillate and finally, the water gets separated. Thus, the liquid can effectively be distilled at a temperature lower than its boiling point.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




