
What is the action of the following reagents on propyne?
(a) Chlorine gas
(b) hydrogen iodide
Answer
513.3k+ views
Hint: Propyne is an alkyne and it is an unsaturated hydrocarbon with the formula ${C_3}{H_4}$. In organic chemistry, a reagent is a compound or substance that is added to a system to cause a chemical reaction or to test if a chemical reaction occurs or not.
Complete answer: (a) When propyne reacts with an excess amount of chlorine gas, hydrochloric acid is formed. Alkynes undergo a halogenation reaction when they react with halogens. The chemical reaction between propyne and chlorine gas takes place in the following way:
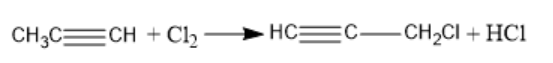
(b) When alkynes react with hydrogen halides, trans-vinyl halides are formed. If the reaction takes place in the presence of excess hydrogen halide, then geminal dihalide is formed. This reaction is an example of electrophilic addition and the major product is determined from the markovnikov’s rule. When an alkyne is treated with one equivalent of hydrogen halide, vinyl halides are formed. When propyne reacts with hydrogen iodide, $2 - {\text{iodopropene}}$ is formed. The chemical reaction between propyne and hydrogen iodide takes place in the following way:
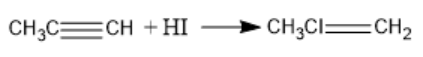
Note:
When alkynes react with hydrogen halides, the major product is predicted by using the Markovnikov’s rule i.e the hydrogen gets attached to the carbon which already has the most number of hydrogen atoms attached to it. This reaction proceeds via a termolecular mechanism. The reaction which involves the simultaneous collision of three ions, atoms or molecules is called a termolecular reaction.
Complete answer: (a) When propyne reacts with an excess amount of chlorine gas, hydrochloric acid is formed. Alkynes undergo a halogenation reaction when they react with halogens. The chemical reaction between propyne and chlorine gas takes place in the following way:

(b) When alkynes react with hydrogen halides, trans-vinyl halides are formed. If the reaction takes place in the presence of excess hydrogen halide, then geminal dihalide is formed. This reaction is an example of electrophilic addition and the major product is determined from the markovnikov’s rule. When an alkyne is treated with one equivalent of hydrogen halide, vinyl halides are formed. When propyne reacts with hydrogen iodide, $2 - {\text{iodopropene}}$ is formed. The chemical reaction between propyne and hydrogen iodide takes place in the following way:
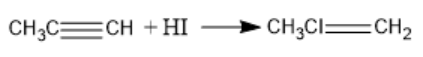
Note:
When alkynes react with hydrogen halides, the major product is predicted by using the Markovnikov’s rule i.e the hydrogen gets attached to the carbon which already has the most number of hydrogen atoms attached to it. This reaction proceeds via a termolecular mechanism. The reaction which involves the simultaneous collision of three ions, atoms or molecules is called a termolecular reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




