
What is the action of sodium nitrate and hydrochloric acid on ethanamine, N-ethylethanamine and N, N-diethylethanamine?
Answer
563.1k+ views
Hint:The reaction of sodium nitrate and hydrochloric acid gives nitrous acid which reacts with amine. Amines work as nucleophiles so they attack on nitronium ions generated by the nitrous acid. The nitronium ions get attached with nitrogen of primary amine. After the removal of hydrogen finally the diazonium salt forms. Diazonium salt is formed by primary amine. In secondary amine the nitrosonium ion directly gets attached with amine’s nitrogen.
Complete solution:
The reaction of amine with sodium nitrate and hydrochloric acid is used for the preparation of diazonium ion.
Ethylamine is a primary amine and N- ethylethanamine is a secondary amine and N, N-diethylethanamine is a tertiary amine. All react differently with nitrous acid.
The product of the reaction of all given amines with nitrous acid is shown as follows:
The reaction of ethylamine with nitrous acid gives ethanol, water and nitrogen gas. Nitrogen is colourless and odourless gas.
The reaction of N- ethylethanamine with nitrous acid gives N-ethylnitrosoethanamine and water.
The tertiary amines do not react with nitrous acid due to absence of hydrogen at nitrogen of amine.
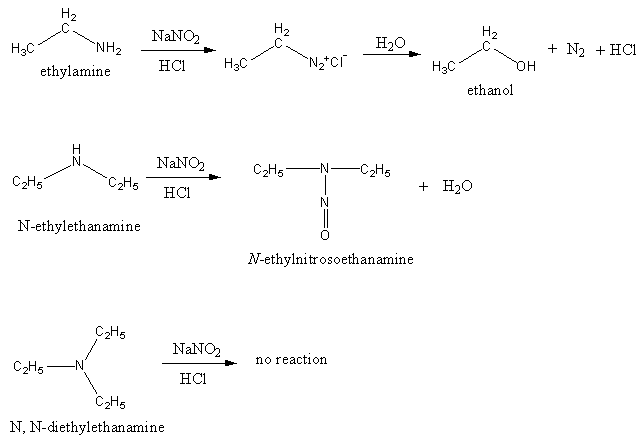
Therefore, primary amines form alcohol. Secondary amines form nitroso compounds. The tertiary amine does not react.
Note:As primary, secondary and tertiary amine reacts differently with nitrous acid so, this reaction is used for the identification of type of amine. By the reaction of primary amine nitrogen gas evolves so, by evolution of the gas, primary amines are identified. The N- methyl nitroso ethanamine (product of secondary amine) is yellow coloured oil. So, secondary amines are identified by the formation of a yellow oil. The amines are basic so, tertiary amine gets protonated in presence of nitrous acid and form cation of a tertiary amine. The nitrous acid is also used for the formation of diazonium salt of amines.
Complete solution:
The reaction of amine with sodium nitrate and hydrochloric acid is used for the preparation of diazonium ion.
Ethylamine is a primary amine and N- ethylethanamine is a secondary amine and N, N-diethylethanamine is a tertiary amine. All react differently with nitrous acid.
The product of the reaction of all given amines with nitrous acid is shown as follows:
The reaction of ethylamine with nitrous acid gives ethanol, water and nitrogen gas. Nitrogen is colourless and odourless gas.
The reaction of N- ethylethanamine with nitrous acid gives N-ethylnitrosoethanamine and water.
The tertiary amines do not react with nitrous acid due to absence of hydrogen at nitrogen of amine.

Therefore, primary amines form alcohol. Secondary amines form nitroso compounds. The tertiary amine does not react.
Note:As primary, secondary and tertiary amine reacts differently with nitrous acid so, this reaction is used for the identification of type of amine. By the reaction of primary amine nitrogen gas evolves so, by evolution of the gas, primary amines are identified. The N- methyl nitroso ethanamine (product of secondary amine) is yellow coloured oil. So, secondary amines are identified by the formation of a yellow oil. The amines are basic so, tertiary amine gets protonated in presence of nitrous acid and form cation of a tertiary amine. The nitrous acid is also used for the formation of diazonium salt of amines.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




