
What is the acidity order of the following compounds?
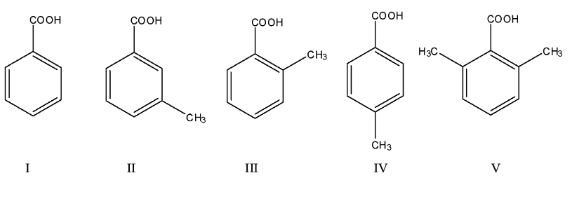
(a)- I > II > III > IV > V
(b)- II > III > IV > V > I
(c)- V > III > I > II > IV
(d)- V > III > II > I > IV

Answer
535.8k+ views
Hint: If the benzoic acid has an electron-donating group, then the stability of the carboxylate ion will decrease, so the acidity will decrease. If the benzoic acid has an electron-withdrawing group, then the stability of the carboxylate ion will increase, so the acidity will increase. But the compound is present on the ortho position, then its acidity will be the highest regardless of the nature of the substituent.
Complete step by step answer: If the benzoic acid has an electron-donating group, then the stability of the carboxylate ion will decrease, so the acidity will decrease. If the benzoic acid has an electron-withdrawing group, then the stability of the carboxylate ion will increase, so the acidity will increase. But the compound is present on the ortho position, then its acidity will be the highest regardless of the nature of the substituent, this is known as ortho-effect.
So, in the compounds given above, there are two compounds in which the methyl group is present at the ortho position, i.e., V and III. Since V has two methyl groups at the ortho position, it will be the highest acidic compound. After that, the III will be the most acidic. Now, we know that a methyl group is an electron-donating group, therefore, II and IV are less acidic than I.
The acidic due to methyl group on the benzoic acid is due to the hyperconjugation effect, the hyperconjugation effect at the p-position has left effect than the meta position, therefore, II will be more acidic than IV.
The order will be V > III > I > II > IV.
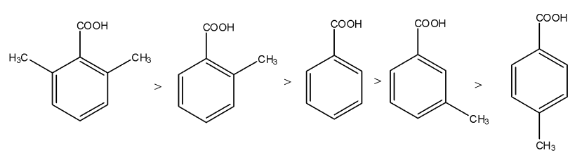
Therefore, the correct answer is an option (c).
Note: If the compounds are not at the ortho position, then the compounds like $N{{O}_{2}}$, Cl, etc will increase the acidity, and compounds like $C{{H}_{3}}$, OH, $OC{{H}_{3}}$, etc will decrease the acidity.
Complete step by step answer: If the benzoic acid has an electron-donating group, then the stability of the carboxylate ion will decrease, so the acidity will decrease. If the benzoic acid has an electron-withdrawing group, then the stability of the carboxylate ion will increase, so the acidity will increase. But the compound is present on the ortho position, then its acidity will be the highest regardless of the nature of the substituent, this is known as ortho-effect.
So, in the compounds given above, there are two compounds in which the methyl group is present at the ortho position, i.e., V and III. Since V has two methyl groups at the ortho position, it will be the highest acidic compound. After that, the III will be the most acidic. Now, we know that a methyl group is an electron-donating group, therefore, II and IV are less acidic than I.
The acidic due to methyl group on the benzoic acid is due to the hyperconjugation effect, the hyperconjugation effect at the p-position has left effect than the meta position, therefore, II will be more acidic than IV.
The order will be V > III > I > II > IV.

Therefore, the correct answer is an option (c).
Note: If the compounds are not at the ortho position, then the compounds like $N{{O}_{2}}$, Cl, etc will increase the acidity, and compounds like $C{{H}_{3}}$, OH, $OC{{H}_{3}}$, etc will decrease the acidity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




