
Acid catalysed Aldol Condensation involves
A. carbanion
B. enolate ion
C. enol
D. both (a) and (c)
Answer
573k+ views
Hint: The main step in Aldol Condensation involves the abstraction of alpha hydrogen that is attached to the carbonyl carbon. It is found that the presence of this proton decides the rate of the reaction.
Complete Solution :
- Let's discuss about the Aldol Condensation reaction:
- We can see that in this reaction, a carbon-carbon bond is formed, in which a $\beta $ –hydroxy ketone or $\beta $ –hydroxy aldehyde is formed. The initiation of this reaction takes place by the formation of an enolate ion using dilute base.
- The enolate ion then attacks a carbonyl carbon leading to the formation of $\beta $ –hydroxy carbonyl compound. We can see the mechanism of Aldol Condensation:
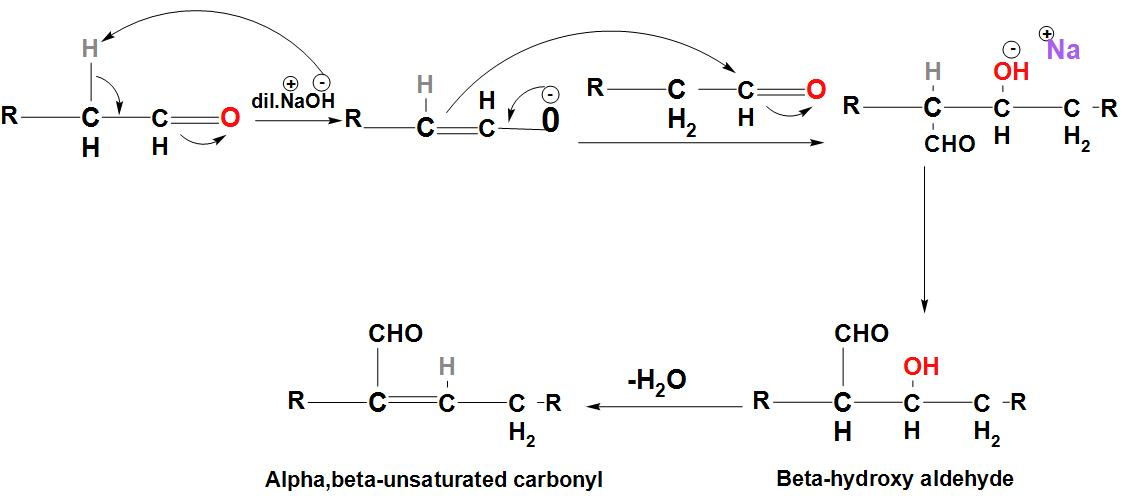
- We can see that dehydration of the $\beta$ –hydroxy aldehyde leads to the formation of –unsaturated aldehyde. We can see here that in the starting material, the hydrogen present at the alpha position is very important. The reaction will not start if there is no hydrogen present at this position.
- Hence, we can conclude that the correct option is (b), that acid catalysed Aldol Condensation involves an enolate ion.
So, the correct answer is “Option B”.
Note: - We should not get confused in between Aldol Condensation and Cannizzaro reaction. As, both of these reactions occur in similar conditions.
- Aldol Condensation occurs in compounds that contain alpha hydrogen. Cannizzaro reaction occurs in compounds that don't contain alpha hydrogen. And carboxylic acid and primary alcohol are formed as products in this reaction.
Complete Solution :
- Let's discuss about the Aldol Condensation reaction:
- We can see that in this reaction, a carbon-carbon bond is formed, in which a $\beta $ –hydroxy ketone or $\beta $ –hydroxy aldehyde is formed. The initiation of this reaction takes place by the formation of an enolate ion using dilute base.
- The enolate ion then attacks a carbonyl carbon leading to the formation of $\beta $ –hydroxy carbonyl compound. We can see the mechanism of Aldol Condensation:

- We can see that dehydration of the $\beta$ –hydroxy aldehyde leads to the formation of –unsaturated aldehyde. We can see here that in the starting material, the hydrogen present at the alpha position is very important. The reaction will not start if there is no hydrogen present at this position.
- Hence, we can conclude that the correct option is (b), that acid catalysed Aldol Condensation involves an enolate ion.
So, the correct answer is “Option B”.
Note: - We should not get confused in between Aldol Condensation and Cannizzaro reaction. As, both of these reactions occur in similar conditions.
- Aldol Condensation occurs in compounds that contain alpha hydrogen. Cannizzaro reaction occurs in compounds that don't contain alpha hydrogen. And carboxylic acid and primary alcohol are formed as products in this reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




