
How is acetylene converted to (i) Westron (ii) Vinyl chloride.
Answer
573.3k+ views
Hint: 1,1,2,2- tetrachloroethane is called westron and it can be prepared from acetylene. Westron and vinyl chloride are prepared from acetylene through addition reaction of acetylene with Chlorine and hydrochloric acid.
Complete Solution :
- In the question it is given how we can prepare Westron and Vinyl chloride from acetylene.
Preparation of Westron from Acetylene:
- The preparation of westron from acetylene is a two-step process.
Step-1:
- In the first step Acetylene reacts with chlorine and forms 1,2 – dichloroethene as the product and chemical reaction of acetylene with chlorine is as follows.
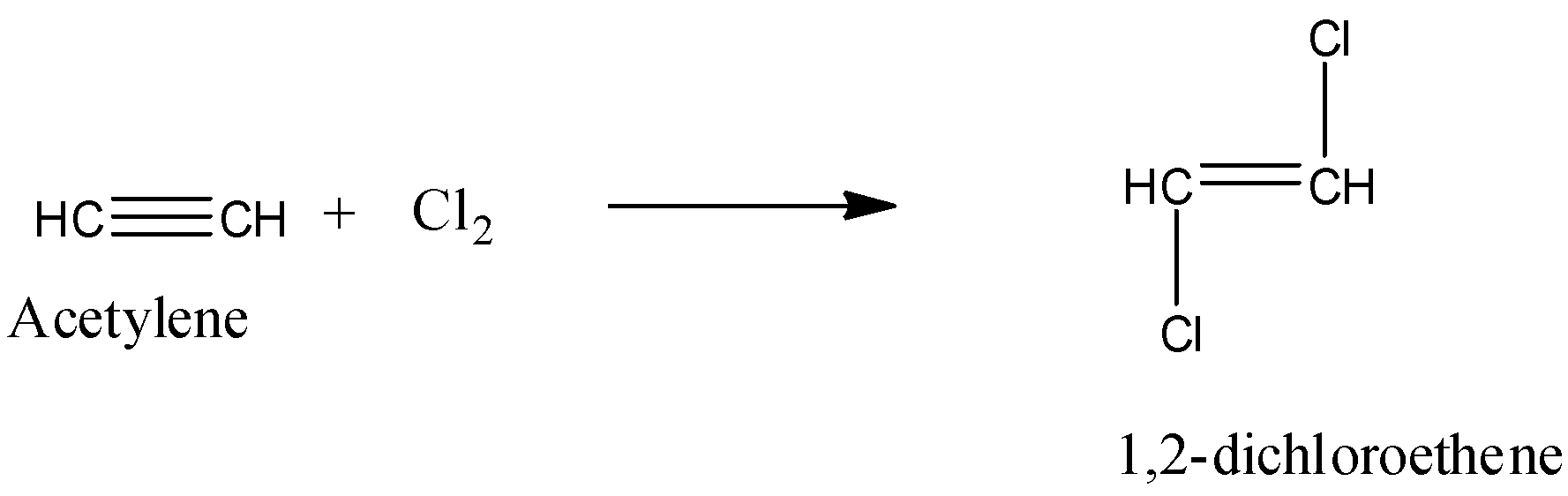
- In the above chemical reaction one mole of acetylene reacts with one mole chlorine and forms one mole of 1,2- dichloroethene as the product.
Step-2:
- In the second step the product formed in the first step is going to react with chlorine and forms Westron (1,1,2,2-tetrachloroethane) as the product.
- The chemical reaction of 1,2- dichloroethene with chlorine is as follows.
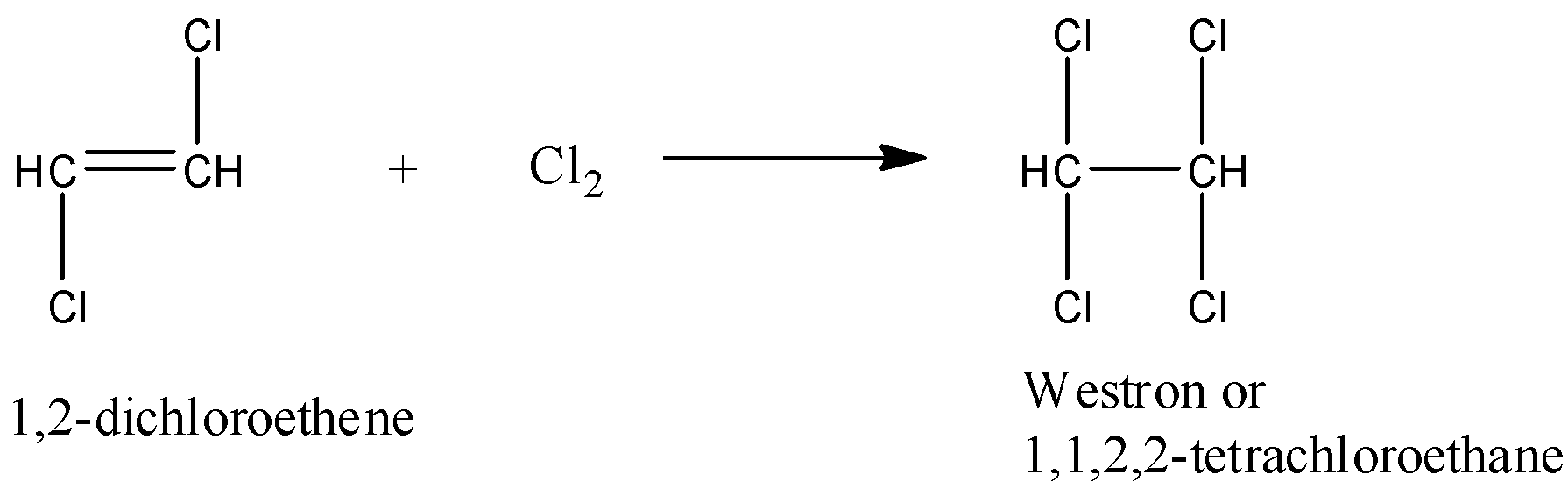
Preparation of vinyl chloride from Acetylene:
- Acetylene reacts with hydrochloric acid and forms vinyl chloride as the product.
- The chemical reaction of acetylene with hydrochloric acid is as follows.
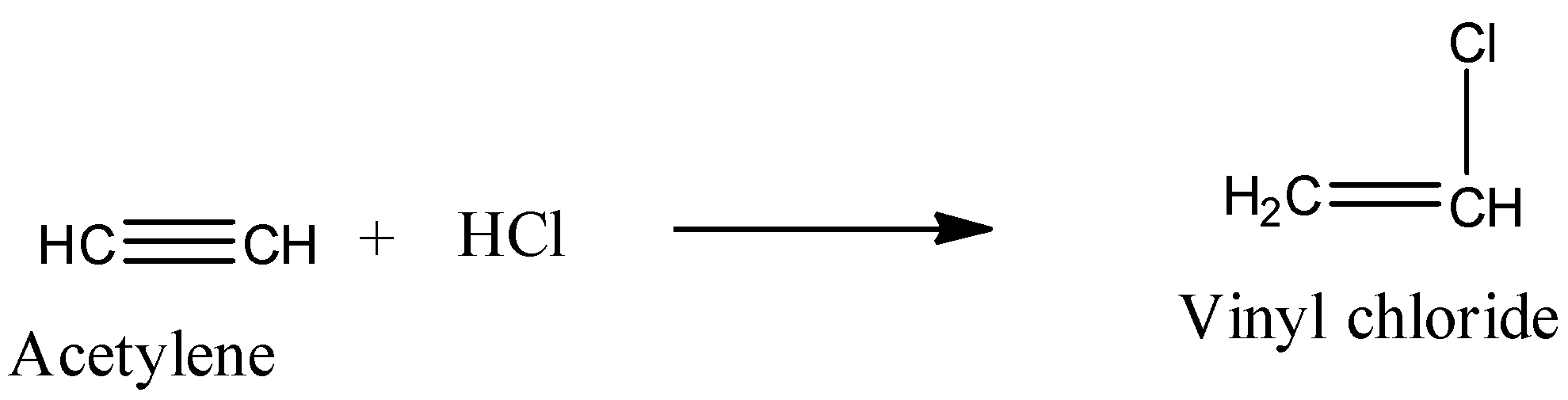
Note: The preparation of westron or vinyl chloride is going to occur through nucleophilic addition reaction. Vinyl chloride further undergoes polymerization reaction and forms polyvinyl chloride (PVC) as the product. PVC has vast applications in various fields. So, acetylene is used in various industries for various purposes.
Complete Solution :
- In the question it is given how we can prepare Westron and Vinyl chloride from acetylene.
Preparation of Westron from Acetylene:
- The preparation of westron from acetylene is a two-step process.
Step-1:
- In the first step Acetylene reacts with chlorine and forms 1,2 – dichloroethene as the product and chemical reaction of acetylene with chlorine is as follows.

- In the above chemical reaction one mole of acetylene reacts with one mole chlorine and forms one mole of 1,2- dichloroethene as the product.
Step-2:
- In the second step the product formed in the first step is going to react with chlorine and forms Westron (1,1,2,2-tetrachloroethane) as the product.
- The chemical reaction of 1,2- dichloroethene with chlorine is as follows.

Preparation of vinyl chloride from Acetylene:
- Acetylene reacts with hydrochloric acid and forms vinyl chloride as the product.
- The chemical reaction of acetylene with hydrochloric acid is as follows.

Note: The preparation of westron or vinyl chloride is going to occur through nucleophilic addition reaction. Vinyl chloride further undergoes polymerization reaction and forms polyvinyl chloride (PVC) as the product. PVC has vast applications in various fields. So, acetylene is used in various industries for various purposes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




