
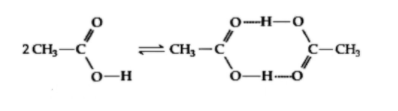
Acetic acid undergoes dimerisation, when dissolved in benzene (as shown in figure)
Molecular mass of acetic acid is found to be 120.
Which among the following relations is correct?
(where, α = degree of association,
d = observed vapour density,
D = Theoretical vapour density).
A. $\alpha \, = \,2\left( {\dfrac{{D - d}}{d}} \right)$
B. $\alpha \, = \,2\left( {\dfrac{{D - d}}{D}} \right)$
C. $\alpha \, = \,2\left( {\dfrac{{d - D}}{d}} \right)$
D. \[\alpha \, = \,\dfrac{{2d}}{{D - d}}\]

Answer
549.6k+ views
Hint: In order to answer this question you must recall the properties of Acetic acid. And recall its reaction with benzene. Acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. And to find the answer you must use the association formula by putting the number of atoms getting associated. And finally you will reach your desired answer.
Complete step by step answer:
Step 1: In this step we will use the association formula, to evaluate the correct relation. And the formula for association is: $\alpha \, = \,\dfrac{{d - D}}{{d\left( {1 - \dfrac{1}{n}} \right)}}$ where n is the number of molecules getting associated. And we know that this is a dimerisation reaction, so by Putting the value n = 2 in the above formula we will calculate the value of α and will reach to our answer :
Therefore, \[\alpha \, = \,\dfrac{{d - D}}{{d\left( {1 - \dfrac{1}{2}} \right)}}\, = \,\dfrac{{d - D}}{{d\left( {\dfrac{1}{2}} \right)}}\, = \,2\left( {\dfrac{{d - D}}{d}} \right)\]
Step 2: Hence we got the value of α, i.e. $\alpha \, = \,2\left( {\dfrac{{d - D}}{d}} \right)$
(where, α = degree of association,
d = observed vapour density,
D = Theoretical vapour density).
The correct answer is option C .
Note: A dimer (di-, "two" + -mer, "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. The term homodimer is used when the two molecules are identical (e.g. A–A) and heterodimer when they are not (e.g. A–B). The reverse of dimerisation is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as Bjerrum pairs.
Complete step by step answer:
Step 1: In this step we will use the association formula, to evaluate the correct relation. And the formula for association is: $\alpha \, = \,\dfrac{{d - D}}{{d\left( {1 - \dfrac{1}{n}} \right)}}$ where n is the number of molecules getting associated. And we know that this is a dimerisation reaction, so by Putting the value n = 2 in the above formula we will calculate the value of α and will reach to our answer :
Therefore, \[\alpha \, = \,\dfrac{{d - D}}{{d\left( {1 - \dfrac{1}{2}} \right)}}\, = \,\dfrac{{d - D}}{{d\left( {\dfrac{1}{2}} \right)}}\, = \,2\left( {\dfrac{{d - D}}{d}} \right)\]
Step 2: Hence we got the value of α, i.e. $\alpha \, = \,2\left( {\dfrac{{d - D}}{d}} \right)$
(where, α = degree of association,
d = observed vapour density,
D = Theoretical vapour density).
The correct answer is option C .
Note: A dimer (di-, "two" + -mer, "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. The term homodimer is used when the two molecules are identical (e.g. A–A) and heterodimer when they are not (e.g. A–B). The reverse of dimerisation is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as Bjerrum pairs.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




