
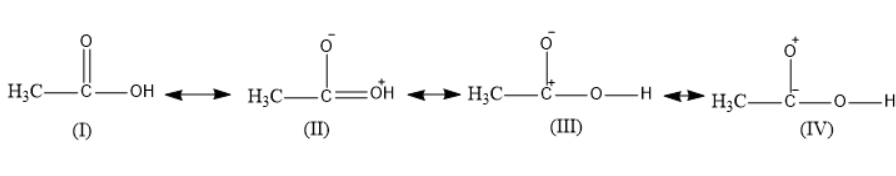
Acetic acid considers as a resonance hybrid of the four structures:
Which of the above is least stable.

Answer
488.4k+ views
Hint: Acetic acid is a weak acid with the molecular formula of \[C{H_3}COOH\] . In acetic acid, the carbonyl oxygen gets a negative charge due to the more electronegativity, whereas carbonyl carbon gets a positive charge. When the carbonyl carbon is a negative charge and oxygen is a positive charge, it will be unstable.
Complete answer:
The resonance structure is the different Lewis structures of the given compound. The charges are distributed in the atoms present in the compound. The atoms, valence electrons, and bonds must be equal in all the resonance structures.
Four resonance structures of acetic acid were given.
The first structure is the normal acetic acid structure.
The second structure represents one of the resonance structures in which the carbonyl oxygen gets a negative charge and the hydroxyl oxygen gets a positive charge.
The third structure represents one of the resonance structures in which the carbonyl oxygen gets a negative charge and the carbonyl carbon gets a positive charge.
The fourth structure represents one of the resonance structures in which the carbonyl carbon gets a negative charge and the carbonyl oxygen gets a positive charge. It is the least unstable as the oxygen is more electronegative; it does not get a positive charge.
Thus, \[IV\] structure is the least stable resonance structure of acetic acid.
Note:
Electronegativity is nothing but the ability to attract the electrons towards themselves. In the periodic table, fluorine is the most electronegative element, followed by oxygen. Oxygen is more electronegative than carbon due to this reason, in the carbonyl group the oxygen atom gets a negative charge only.
Complete answer:
The resonance structure is the different Lewis structures of the given compound. The charges are distributed in the atoms present in the compound. The atoms, valence electrons, and bonds must be equal in all the resonance structures.
Four resonance structures of acetic acid were given.
The first structure is the normal acetic acid structure.
The second structure represents one of the resonance structures in which the carbonyl oxygen gets a negative charge and the hydroxyl oxygen gets a positive charge.
The third structure represents one of the resonance structures in which the carbonyl oxygen gets a negative charge and the carbonyl carbon gets a positive charge.
The fourth structure represents one of the resonance structures in which the carbonyl carbon gets a negative charge and the carbonyl oxygen gets a positive charge. It is the least unstable as the oxygen is more electronegative; it does not get a positive charge.
Thus, \[IV\] structure is the least stable resonance structure of acetic acid.
Note:
Electronegativity is nothing but the ability to attract the electrons towards themselves. In the periodic table, fluorine is the most electronegative element, followed by oxygen. Oxygen is more electronegative than carbon due to this reason, in the carbonyl group the oxygen atom gets a negative charge only.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




