
Acetate ion contains :
a.) One C, O single bond and one C, O double bond
b.) Two C, O single bond
c.) Two C, O double bond
d.) None of these
Answer
561k+ views
Hint The ethanoic acid is also known as acetic acid. When a Hydrogen is abstracted from acetic acid, the acetate ion is formed. It is chemically written as - $C{H_3}CO{O^ - }$. So, the number of bonds can be seen from the above formula.
Complete answer :
We know that ethanoic acid is also known as acetic acid. When a Hydrogen is abstracted from acetic acid, the acetate ion is formed. It is chemically written as - $C{H_3}CO{O^ - }$.
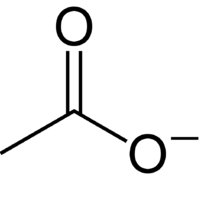
So, it has one C-C single bond, three C-H single bonds, one C-O single bond and one C-O double bond.
So, it contains one C-O single bond and one C-O double bond.
Thus, the option a is the correct answer.
Note : It must be noted that acetate is a monocarboxylic acid anion that formed after removal of a proton from acetic acid. It is a common anion in biology that is used by organisms in the form of acetyl coenzyme A. Acetic acid can undergo a dissmutation reaction to form methane and carbon dioxide. It is an important building block for biosynthesis. These acetic acids combine to give more molecules like fatty acids. The acetic acid is an important chemical used in industries. It is used as a polar protic solvent. It is used as an effective antiseptic.
Complete answer :
We know that ethanoic acid is also known as acetic acid. When a Hydrogen is abstracted from acetic acid, the acetate ion is formed. It is chemically written as - $C{H_3}CO{O^ - }$.

So, it has one C-C single bond, three C-H single bonds, one C-O single bond and one C-O double bond.
So, it contains one C-O single bond and one C-O double bond.
Thus, the option a is the correct answer.
Note : It must be noted that acetate is a monocarboxylic acid anion that formed after removal of a proton from acetic acid. It is a common anion in biology that is used by organisms in the form of acetyl coenzyme A. Acetic acid can undergo a dissmutation reaction to form methane and carbon dioxide. It is an important building block for biosynthesis. These acetic acids combine to give more molecules like fatty acids. The acetic acid is an important chemical used in industries. It is used as a polar protic solvent. It is used as an effective antiseptic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




