
When acetaldehyde is reacted with \[LiAl{{H}_{4}}\], What is the product formed?
A.\[C{{H}_{3}}COOH\]
B.\[C{{H}_{3}}C{{H}_{2}}OH\]
C.\[C{{H}_{3}}OH\]
D.\[HCOOH\]
Answer
493.2k+ views
Hint: The molecular formula of acetaldehyde is \[C{{H}_{3}}CHO\]. Lithium aluminium hydride \[(LiAl{{H}_{4}})\] is a reducing agent which facilitates the reduction of the reacting substrate. Reduction describes the addition of hydrogen.
Complete answer:
The molecular formula of acetaldehyde is \[C{{H}_{3}}CHO\]. Lithium aluminium hydride \[(LiAl{{H}_{4}})\] is a reducing agent which facilitates the reduction of the reacting substrate. Reduction describes the addition of hydrogen. Thus, the reduction of aldehyde in presence of \[LiAl{{H}_{4}}\] lead to the formation of alcohol. This is illustrated in the following reaction:
\[C{{H}_{3}}CHO+LiAl{{H}_{4}}\to C{{H}_{3}}C{{H}_{2}}OH\]
The general reaction mechanism for the above reduction is given as:
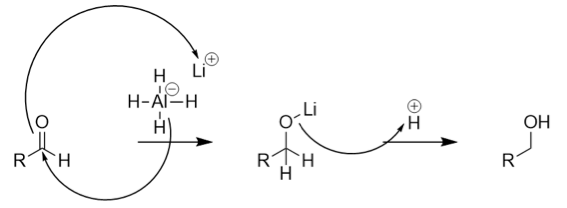
From the above mechanism, we conclude that the reduction of aldehyde takes place via hydride transfer. Upon reduction, the number of carbon atoms in the moiety remains the same. Therefore, option (B) is correct. In contrast, option (A) contains \[C{{H}_{3}}COOH\] which is the oxidised form of acetaldehyde thus this option is incorrect. Option (C) contains \[C{{H}_{3}}OH\] which contains less number of the carbon atoms than acetaldehyde. Thus, option (A) is incorrect. Option (D) contains \[HCOOH\] which is the oxidised form of formaldehyde instead of acetaldehyde. Thus, option (D) is incorrect.
From the above discussion, we conclude that option (B) is correct.
Note:
It is important to note that acetaldehyde undergoes reduction when reacted with \[LiAl{{H}_{4}}\]. This reduction takes place via hydride transfer. The final product will be \[C{{H}_{3}}C{{H}_{2}}OH\] and hence option (B) is correct.
Complete answer:
The molecular formula of acetaldehyde is \[C{{H}_{3}}CHO\]. Lithium aluminium hydride \[(LiAl{{H}_{4}})\] is a reducing agent which facilitates the reduction of the reacting substrate. Reduction describes the addition of hydrogen. Thus, the reduction of aldehyde in presence of \[LiAl{{H}_{4}}\] lead to the formation of alcohol. This is illustrated in the following reaction:
\[C{{H}_{3}}CHO+LiAl{{H}_{4}}\to C{{H}_{3}}C{{H}_{2}}OH\]
The general reaction mechanism for the above reduction is given as:

From the above mechanism, we conclude that the reduction of aldehyde takes place via hydride transfer. Upon reduction, the number of carbon atoms in the moiety remains the same. Therefore, option (B) is correct. In contrast, option (A) contains \[C{{H}_{3}}COOH\] which is the oxidised form of acetaldehyde thus this option is incorrect. Option (C) contains \[C{{H}_{3}}OH\] which contains less number of the carbon atoms than acetaldehyde. Thus, option (A) is incorrect. Option (D) contains \[HCOOH\] which is the oxidised form of formaldehyde instead of acetaldehyde. Thus, option (D) is incorrect.
From the above discussion, we conclude that option (B) is correct.
Note:
It is important to note that acetaldehyde undergoes reduction when reacted with \[LiAl{{H}_{4}}\]. This reduction takes place via hydride transfer. The final product will be \[C{{H}_{3}}C{{H}_{2}}OH\] and hence option (B) is correct.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




