
According to VSEPR theory, it predicts the geometry and shape of \[{I_3}^ + \] ions.
Answer
508.5k+ views
Hint: VSEPR theory gives the basic idea that bonded atoms in a molecule adopt that particular arrangement in the space around the central atom which keeps them on an average as far apart as possible.
VSEPR theory gives the idea of shape and geometry of atoms around the central atom to attain stable configuration.
Complete answer:
As we know iodine is member of halogen family with valence shell electron configuration $\left[ {Kr} \right]4{d^{10}}5{s^2}5{p^5}$
Hence, total number of valence electron in iodine atom is $\left( 7 \right)$s
In the given situation iodine present in its positive ions by losing an electron from its valence shell.so final electronic configuration become $\left[ {Kr} \right]4{d^{10}}5{s^2}5{p^4}$
All the three atoms of iodine attain the same electronic configuration with a total number of $\left( 6 \right)$ electrons.
Out of three iodine atoms one atom acts as the central atom while the other two act as the side atom .
Formula of calculating hybridization of molecules is
$H = \dfrac{1}{2}\left[ {G + M - C + A} \right]$
Where, $\left( H \right)$ is hybridization of molecule
$G$ is valence shell electron of central atom which is iodine in this case
$M$ Is number of monovalent atoms attached directly to central atoms like two iodine atoms attached to central iodine
$C$ Is total value of cationic charge present in molecule
$A$ Is total value of anionic charge present in molecule
Now put all the values in the above equation we get
$H = \dfrac{1}{2}\left[ {7 + 2 - 1 + 0} \right]$
$H = \dfrac{1}{2}\left( 8 \right)$
After solving above equation we get
$H = 4$
Hence, hybridization of molecules is $s{p^3}$ according to VSEPR theory.
In general the geometry of hybridization is trigonal planar but presence of charge over the central atom $\left( I \right)$. It forces the bond to bend away from the central atom to arrange into stable configuration.
Due to more lone pair -bond pair repulsion in the molecule, bonds are pushed more towards each other and it results in reduction of bond angle from ${119.5^ \circ }$ to ${120^ \circ }$.
This arrangement leads to development of V- shaped geometry of molecules.
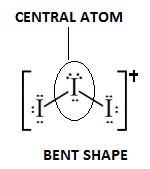
Finally we conclude that according to VSEPR theory the geometry of \[{I_3}^ + \] ion is $s{p^3}$ and the shape is Bent or V- shaped.
Note:
In the formula of hybridization total electrons in the valence shell of the central atom are placed while during the presentation the total number of the electrons left in central atoms are shown.
Other molecules show bent shape geometry are- ${H_2}O,S{O_2}$.
VSEPR theory gives the idea of shape and geometry of atoms around the central atom to attain stable configuration.
Complete answer:
As we know iodine is member of halogen family with valence shell electron configuration $\left[ {Kr} \right]4{d^{10}}5{s^2}5{p^5}$
Hence, total number of valence electron in iodine atom is $\left( 7 \right)$s
In the given situation iodine present in its positive ions by losing an electron from its valence shell.so final electronic configuration become $\left[ {Kr} \right]4{d^{10}}5{s^2}5{p^4}$
All the three atoms of iodine attain the same electronic configuration with a total number of $\left( 6 \right)$ electrons.
Out of three iodine atoms one atom acts as the central atom while the other two act as the side atom .
Formula of calculating hybridization of molecules is
$H = \dfrac{1}{2}\left[ {G + M - C + A} \right]$
Where, $\left( H \right)$ is hybridization of molecule
$G$ is valence shell electron of central atom which is iodine in this case
$M$ Is number of monovalent atoms attached directly to central atoms like two iodine atoms attached to central iodine
$C$ Is total value of cationic charge present in molecule
$A$ Is total value of anionic charge present in molecule
Now put all the values in the above equation we get
$H = \dfrac{1}{2}\left[ {7 + 2 - 1 + 0} \right]$
$H = \dfrac{1}{2}\left( 8 \right)$
After solving above equation we get
$H = 4$
Hence, hybridization of molecules is $s{p^3}$ according to VSEPR theory.
In general the geometry of hybridization is trigonal planar but presence of charge over the central atom $\left( I \right)$. It forces the bond to bend away from the central atom to arrange into stable configuration.
Due to more lone pair -bond pair repulsion in the molecule, bonds are pushed more towards each other and it results in reduction of bond angle from ${119.5^ \circ }$ to ${120^ \circ }$.
This arrangement leads to development of V- shaped geometry of molecules.

Finally we conclude that according to VSEPR theory the geometry of \[{I_3}^ + \] ion is $s{p^3}$ and the shape is Bent or V- shaped.
Note:
In the formula of hybridization total electrons in the valence shell of the central atom are placed while during the presentation the total number of the electrons left in central atoms are shown.
Other molecules show bent shape geometry are- ${H_2}O,S{O_2}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




