
According to the valence bond theory, the predicted bond angle for $H_2O$ is:
A.$90^o$
B.$109^o28$
C.$107^o18$
D.$104^o28$
Answer
564.6k+ views
Hint: We need to know what is valence bond theory and how is it used to predict the bond angles of various molecules. Valence Bond theory is used to describe the covalent bond formation along with the electronic structure of molecules. The theory assumes that electrons in a molecule occupy atomic orbitals rather than molecular orbitals. The atomic orbitals overlap on the bond formation and the larger the overlap the stronger the bond.
Complete step by step answer:
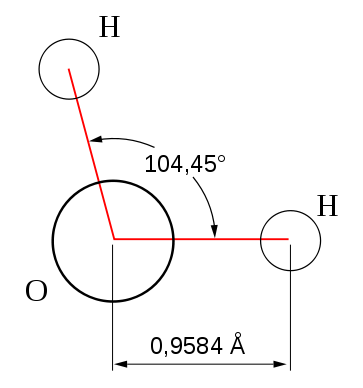
We must remember that an important concept under valence bond theory is Hybridisation which is used to explain the characteristic geometric shapes of polyatomic molecules. According to this concept, the atomic orbitals combine to form a new set of equivalent orbitals known as hybrid orbitals which are used in bond formation and the phenomenon is known as hybridisation. There are various types of hybridisation involving s, p, and d orbitals. In case of ${H_2}O$ , the four oxygen orbitals(one 2s and three 2p) undergo $s{p^3}$ type of hybridisation forming four $sp^3$ orbitals out of which two contain one electron each and the other two contain a pair of electrons. These orbitals are directed towards the four corners of a tetrahedron with an angle of $109^\circ 28$ .However, two corners are occupied by two hydrogens and the other two are occupied by a lone pair of electrons each hence the bond angle is reduced to $104^\circ 28$ . We can draw the structure of water molecule as,
Hence, the correct option is option (D).
Note:
We know that Molecular Orbital (MO) theory is good for understanding bonding in general although it is a difficult concept, but useful in predicting the actual properties of molecules better than VB theory. Also, according to the VSEPR theory, the repulsive interactions of electron pairs decrease in the order: Lone pair-Lone pair > Lone pair-Bond pair > Bond pair-Bond pair. Since water has 2 lone pairs, the bond angle is predicted to be $104^o28$.
Complete step by step answer:
We must remember that an important concept under valence bond theory is Hybridisation which is used to explain the characteristic geometric shapes of polyatomic molecules. According to this concept, the atomic orbitals combine to form a new set of equivalent orbitals known as hybrid orbitals which are used in bond formation and the phenomenon is known as hybridisation. There are various types of hybridisation involving s, p, and d orbitals. In case of ${H_2}O$ , the four oxygen orbitals(one 2s and three 2p) undergo $s{p^3}$ type of hybridisation forming four $sp^3$ orbitals out of which two contain one electron each and the other two contain a pair of electrons. These orbitals are directed towards the four corners of a tetrahedron with an angle of $109^\circ 28$ .However, two corners are occupied by two hydrogens and the other two are occupied by a lone pair of electrons each hence the bond angle is reduced to $104^\circ 28$ . We can draw the structure of water molecule as,

Hence, the correct option is option (D).
Note:
We know that Molecular Orbital (MO) theory is good for understanding bonding in general although it is a difficult concept, but useful in predicting the actual properties of molecules better than VB theory. Also, according to the VSEPR theory, the repulsive interactions of electron pairs decrease in the order: Lone pair-Lone pair > Lone pair-Bond pair > Bond pair-Bond pair. Since water has 2 lone pairs, the bond angle is predicted to be $104^o28$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




