
According to IUPAC convention, name of the following compound is:
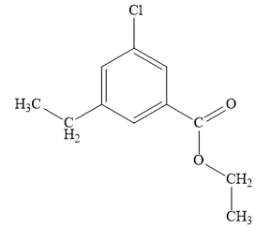
A. 3-chloro-5-ethyl benzoate
B. ethyl-3-chloro-5-ethyl benzoate
C. meta chloro-meta methyl ethyl ethyl benzoate
D. both 'B' and 'C' are correct

Answer
569.4k+ views
Hint:Identify the parent compound and the substituents present. Identify the order of priority of groups for numbering the benzene ring.
Complete answer:
The option A is incorrect because it says that the alkyl part of the alcohol from which benzoate easter was obtained, contains chloro substituent which is incorrect.
The option C is incorrect because in the IUPAC system, the prefix meta is not used. Instead the carbon atoms of benzene ring are numbered. So, you can rule out both options C and D.
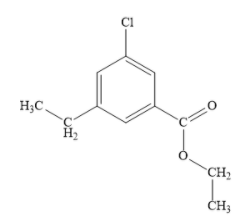
Draw the structure of given organic compound as shown below:
In the above compound, the parent compound is ethyl benzoate. Write the structure of parent compound as shown below:
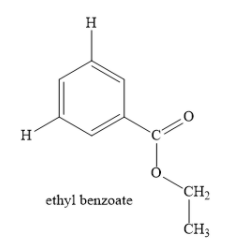
In the parent compound, ethyl ester of benzoic acid is present.
The given compound is the derivative of ethyl benzoate with two substituents on the benzene ring, one is chlorine atom and other is ethyl group.
You can start the numbering of the benzene ring from the carbon atom bearing ester substituent and then go in the direction that gives the lowest locant to the chlorine atom.
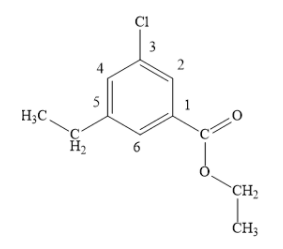
The correct IUPAC name of the given compound is ethyl 3-chloro-5-ethyl benzoate.
Hence, the correct option is the option B.
Note:
Benzoic acid contains a carboxyl group linked to benzene rings. When it reacts with ethanol in presence of sulphuric acid catalyst, ethyl benzoate is obtained.
Complete answer:
The option A is incorrect because it says that the alkyl part of the alcohol from which benzoate easter was obtained, contains chloro substituent which is incorrect.
The option C is incorrect because in the IUPAC system, the prefix meta is not used. Instead the carbon atoms of benzene ring are numbered. So, you can rule out both options C and D.
Draw the structure of given organic compound as shown below:

In the above compound, the parent compound is ethyl benzoate. Write the structure of parent compound as shown below:

In the parent compound, ethyl ester of benzoic acid is present.
The given compound is the derivative of ethyl benzoate with two substituents on the benzene ring, one is chlorine atom and other is ethyl group.
You can start the numbering of the benzene ring from the carbon atom bearing ester substituent and then go in the direction that gives the lowest locant to the chlorine atom.

The correct IUPAC name of the given compound is ethyl 3-chloro-5-ethyl benzoate.
Hence, the correct option is the option B.
Note:
Benzoic acid contains a carboxyl group linked to benzene rings. When it reacts with ethanol in presence of sulphuric acid catalyst, ethyl benzoate is obtained.
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




