
Accomplish the following conversions:
(i) Nitrobenzene to benzoic acid
Answer
566.7k+ views
Hint: The answer to this question is based on the basic concepts of organic chemistry which includes the reagents used for particular conversions and also the type of reaction that is whether oxidation, reduction or substitution reaction.
Complete step by step answer:
In our classes in organic chemistry, we have studied the named reactions and also several common reactions that include addition reaction, substitution reactions, elimination reactions and so on.
To convert nitrobenzene to benzoic acid, firstly we must know that nitro group is electron withdrawing group and thus it is reduced in first step to aniline with amine group in it in the presence of reducing agent that is \[{{H}_{2}}/Pd\] and then converting it into diazonium salt which when treated with copper cyanide and subsequent hydrolysis yields benzoic acid. The reaction is shown below,
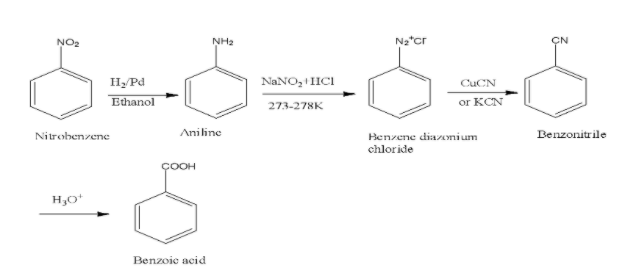
Note: Note that several common oxidising and reducing agents are to be remembered and based on this you will be able to solve any type of such question that involve conversions from one compound to another.
Complete step by step answer:
In our classes in organic chemistry, we have studied the named reactions and also several common reactions that include addition reaction, substitution reactions, elimination reactions and so on.
To convert nitrobenzene to benzoic acid, firstly we must know that nitro group is electron withdrawing group and thus it is reduced in first step to aniline with amine group in it in the presence of reducing agent that is \[{{H}_{2}}/Pd\] and then converting it into diazonium salt which when treated with copper cyanide and subsequent hydrolysis yields benzoic acid. The reaction is shown below,

Note: Note that several common oxidising and reducing agents are to be remembered and based on this you will be able to solve any type of such question that involve conversions from one compound to another.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




