
(a) write the IUPAC name and structural formula of methyl n- propyl ether.
(b) give equations for preparations of ether by the following reactions:
(i) dehydration of alcohols
(ii) Williamson synthesis
(c) Write the resonance structures of alkoxy benzene.
Answer
531.9k+ views
Hint: IUPAC has made certain norms and standards for naming of compounds. Ethers can be prepared by the removal of water from alcohol. Resonance structures are the possible forms of a compound that have no real existence. Alkoxy benzene is a form of ether.
Complete answer:
(a)The IUPAC name of methyl n- propyl ether is 1-methoxy propane. It consists of an ether and around this group one methyl and one propyl group, hence its name. the structure of methyl n- propyl ether is,
(b) ethers are prepared by 2 methods,
(i) dehydration of alcohol.
Ethers are prepared by the alcohols, treated with concentrated sulphuric acid at high temperature which acts as a dehydrating agent and removes water molecules. The reaction is:
$C{{H}_{3}}C{{H}_{2}}OH\xrightarrow[413K]{conc.{{H}_{2}}S{{O}_{4}}}C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{3}}$
Ethanol on dehydration gives ethoxy ethane.
(ii) Williamson synthesis
This method is the lab method to prepare ethers. It involves the reaction between an alkyl halide and sodium alkoxide to obtain ethers. The reaction followed here is ${{S}_{N}}^{2}$ mechanism for a better yield. The reaction is:
${{C}_{2}}{{H}_{5}}Br+{{C}_{2}}{{H}_{5}}ONa\to {{C}_{2}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}+NaBr$
Bromoethane reacts with sodium ethoxide to form ethoxy ethane and sodium bromide.
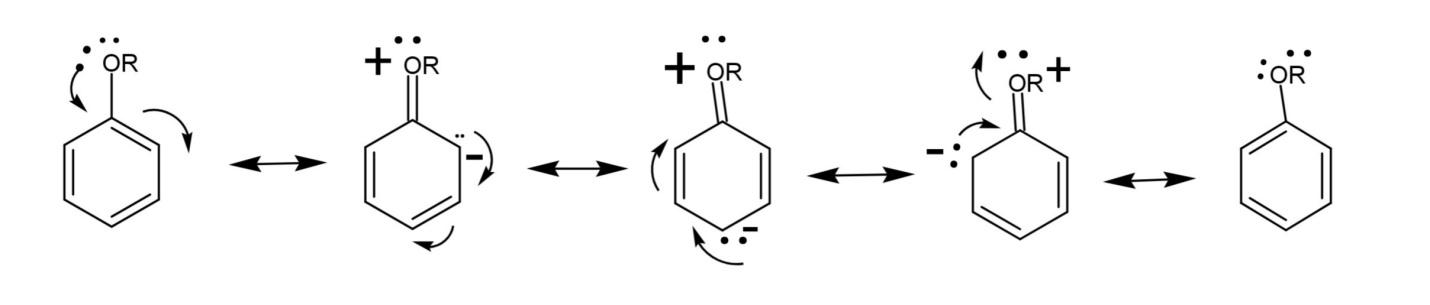
(c) Resonance structures of alkoxy benzene.
Alkoxy benzene has a benzene ring attached with an alkyl group and oxygen, forming an ether. The lone pair on oxygen is in conjugation with the ring, the oxygen atom acquires positive charge, creating partial negative charges on ortho and para positions. The resonance structures are:
Note:
Williamson synthesis is employed to form unsymmetrical as well as symmetrical ethers. When sodium alkoxide used can be of $1{}^\circ ,2{}^\circ ,$ or $3{}^\circ $. For preparing aromatic ethers only sodium phenoxide is used as the reactant. In dehydration of alcohol, the substitution of the alkyl chain from alcohol happens to create the alkyl part of ethers.
Complete answer:
(a)The IUPAC name of methyl n- propyl ether is 1-methoxy propane. It consists of an ether and around this group one methyl and one propyl group, hence its name. the structure of methyl n- propyl ether is,

(b) ethers are prepared by 2 methods,
(i) dehydration of alcohol.
Ethers are prepared by the alcohols, treated with concentrated sulphuric acid at high temperature which acts as a dehydrating agent and removes water molecules. The reaction is:
$C{{H}_{3}}C{{H}_{2}}OH\xrightarrow[413K]{conc.{{H}_{2}}S{{O}_{4}}}C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{3}}$
Ethanol on dehydration gives ethoxy ethane.
(ii) Williamson synthesis
This method is the lab method to prepare ethers. It involves the reaction between an alkyl halide and sodium alkoxide to obtain ethers. The reaction followed here is ${{S}_{N}}^{2}$ mechanism for a better yield. The reaction is:
${{C}_{2}}{{H}_{5}}Br+{{C}_{2}}{{H}_{5}}ONa\to {{C}_{2}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}+NaBr$
Bromoethane reacts with sodium ethoxide to form ethoxy ethane and sodium bromide.
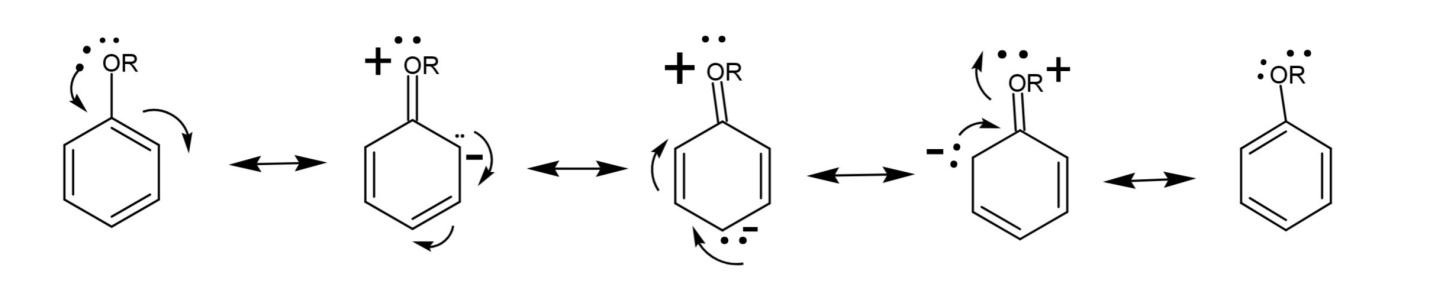
(c) Resonance structures of alkoxy benzene.
Alkoxy benzene has a benzene ring attached with an alkyl group and oxygen, forming an ether. The lone pair on oxygen is in conjugation with the ring, the oxygen atom acquires positive charge, creating partial negative charges on ortho and para positions. The resonance structures are:

Note:
Williamson synthesis is employed to form unsymmetrical as well as symmetrical ethers. When sodium alkoxide used can be of $1{}^\circ ,2{}^\circ ,$ or $3{}^\circ $. For preparing aromatic ethers only sodium phenoxide is used as the reactant. In dehydration of alcohol, the substitution of the alkyl chain from alcohol happens to create the alkyl part of ethers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




