
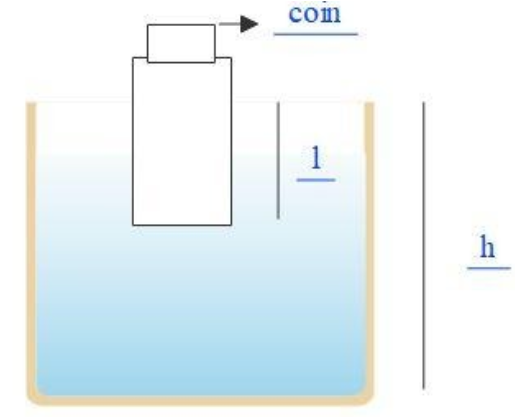
A wooden block with a coin placed at its top, floats in water as shown in the figure. The distance l and h are shown there. After some time, the coin falls into water, then,
Answer
587.4k+ views
Hint:Check the relation between the densities of wood, water, and the coin. Considering the densities of the three materials, we will get to know that the density of the coin is greater than the densities of wood and water.
Complete step-by-step solution:
The coin is placed on the top of the wooden block. As the density of the coin is much greater, it applies a much greater force on the wood before falling into the water. Once it falls off the wood, the force that is exerted by the coin over the wood decreases, which makes the wood go slightly upper on the water. Thus, the value l decreases. Next, we know, the density is inversely proportional to the volume of the object. As the density of the coin is very much higher, its volume will be comparatively lower. Therefore, when the coin falls down, the wood goes up, which means, the volume of the liquid displaced will decrease too. The falling of the coin will not affect the liquid much. Therefore, as a result of both the displacements, the value of h will also decrease.
Additional information:
Density, the weight of an object divided by its volume, is a property of all matter including solids, liquids, and gases. The density of an object depends on what it’s made of and it also depends on at what temperature it is made. Density doesn’t depend on how big it is, the size doesn’t tell us about the density. All the sizes made up of the same substance have the same density.
Note: Density is an intrinsic property, it doesn’t depend on the size or shape. Density depends on many factors such as pressure or temperature. Usually, if the temperature decreases, the density increases, and if temperature increases, density decreases. Water is a bit different. When water freezes, its density decreases. This behavior is called an anomalous behavior of water.
Note: Density is an intrinsic property, it doesn’t depend on the size or shape. Density depends on many factors such as pressure or temperature. Usually, if the temperature decreases, the density increases, and if temperature increases, density decreases. Water is a bit different. When water freezes, its density decreases. This behavior is called an anomalous behavior of water.
Complete step-by-step solution:
The coin is placed on the top of the wooden block. As the density of the coin is much greater, it applies a much greater force on the wood before falling into the water. Once it falls off the wood, the force that is exerted by the coin over the wood decreases, which makes the wood go slightly upper on the water. Thus, the value l decreases. Next, we know, the density is inversely proportional to the volume of the object. As the density of the coin is very much higher, its volume will be comparatively lower. Therefore, when the coin falls down, the wood goes up, which means, the volume of the liquid displaced will decrease too. The falling of the coin will not affect the liquid much. Therefore, as a result of both the displacements, the value of h will also decrease.

Additional information:
Density, the weight of an object divided by its volume, is a property of all matter including solids, liquids, and gases. The density of an object depends on what it’s made of and it also depends on at what temperature it is made. Density doesn’t depend on how big it is, the size doesn’t tell us about the density. All the sizes made up of the same substance have the same density.
Note: Density is an intrinsic property, it doesn’t depend on the size or shape. Density depends on many factors such as pressure or temperature. Usually, if the temperature decreases, the density increases, and if temperature increases, density decreases. Water is a bit different. When water freezes, its density decreases. This behavior is called an anomalous behavior of water.
Note: Density is an intrinsic property, it doesn’t depend on the size or shape. Density depends on many factors such as pressure or temperature. Usually, if the temperature decreases, the density increases, and if temperature increases, density decreases. Water is a bit different. When water freezes, its density decreases. This behavior is called an anomalous behavior of water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




