
a) Which type of an emulsion is 'vanishing cream'. Write its appropriate name.
b) Draw a neat and labelled diagram of above emulsion.
Answer
583.2k+ views
Hint: Identify the dispersed phase and dispersion medium present in vanishing cream. From this you can identify the type of emulsion it is and the appropriate name for it. After that based on your understanding you can draw a diagram showing the dispersed phase and dispersion medium. Do keep in mind that emulsions can be again of two types, namely:
- Oil in water emulsion
- Water in oil emulsion
Complete step by step answer:
An emulsion is a mixture of more than one type of liquids that are generally immiscible( unmixable) due to its liquid-liquid phase separation.
Emulsions are a part of a general class of two-phase systems called colloids. However, emulsion is a type of colloid when the dispersed phase as well as dispersed medium are liquids only.
Two liquids can form two different types of emulsions based on their role in the emulsion. An oil in water emulsion has oil as the dispersed phase and water as the continuous medium or dispersed medium. On the other hand, water in oil type of emulsion has water as the dispersed phase and oil as dispersion medium.
(a)Vanishing cream is a cream or ointment that leaves no trace when rubbed in to skin or moisture. It is an example of oil in water type of emulsion.
The main constituents of this cream are potassium, ammonium or sodium stearate with water and just free stearic acid.
It also contains glycerol and small amounts of fatty ingredients which are hygroscopic in nature.
(b) A diagram is given below depicting the structure of vanishing cream.
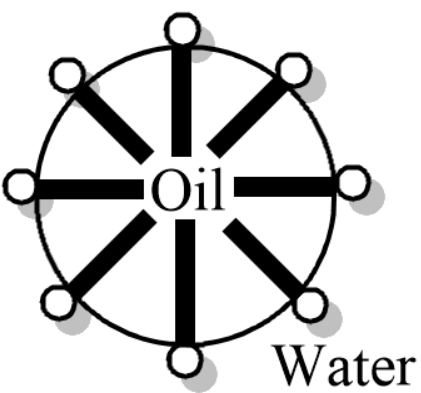
Note: Emulsion stability refers to the ability of an emulsion to resist change in its composition or properties over time or upon long standing. Vanishing cream is a stable emulsion due to a chemical added to it called emulsifiers. Emulsifiers make an emulsion stable by bonding with the water soluble and water insoluble part thus making the liquid-liquid colloidal system stable.
- Oil in water emulsion
- Water in oil emulsion
Complete step by step answer:
An emulsion is a mixture of more than one type of liquids that are generally immiscible( unmixable) due to its liquid-liquid phase separation.
Emulsions are a part of a general class of two-phase systems called colloids. However, emulsion is a type of colloid when the dispersed phase as well as dispersed medium are liquids only.
Two liquids can form two different types of emulsions based on their role in the emulsion. An oil in water emulsion has oil as the dispersed phase and water as the continuous medium or dispersed medium. On the other hand, water in oil type of emulsion has water as the dispersed phase and oil as dispersion medium.
(a)Vanishing cream is a cream or ointment that leaves no trace when rubbed in to skin or moisture. It is an example of oil in water type of emulsion.
The main constituents of this cream are potassium, ammonium or sodium stearate with water and just free stearic acid.
It also contains glycerol and small amounts of fatty ingredients which are hygroscopic in nature.
(b) A diagram is given below depicting the structure of vanishing cream.

Note: Emulsion stability refers to the ability of an emulsion to resist change in its composition or properties over time or upon long standing. Vanishing cream is a stable emulsion due to a chemical added to it called emulsifiers. Emulsifiers make an emulsion stable by bonding with the water soluble and water insoluble part thus making the liquid-liquid colloidal system stable.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




