
A) Which alkane would you expect to get by the action of water on n-propyl magnesium chloride?
B) On isopropyl magnesium chloride?
Answer
512.1k+ views
Hint: Grignard reagent is one of the organometallic compounds. Organometallic compound means that the compound has a carbon metal direct bond. In Grignard reagent all are having a divalent metal. Grignard reagent almost having in magnesium metal only. The bond between carbon atom and magnesium metal is normally covalent only but highly polar. In organic chemistry, carbon are mostly less electronegativity, but compare with metal it having more electronegativity. That the reason here carbon having partial negative charge and magnesium having partial positive charge.
Complete answer:
The given data is
The action of water on n-propyl magnesium chloride to give as,
The molecular formula of n-propyl magnesium chloride is \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - Mg - Cl}}\]
The molecular formula of water is \[{{\text{H}}_{\text{2}}}{\text{O}}\].
The reaction of water on n-propyl magnesium chloride is given below,
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - Mg - Cl + }}{{\text{H}}_{\text{2}}}{\text{O}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{3}}} + {\text{HO - Mg - Cl}}\]
The action of water on n-propyl magnesium chloride is given as n-propane.
The action of water on isopropyl magnesium chloride to give as,
The molecular formula of isopropyl magnesium chloride is
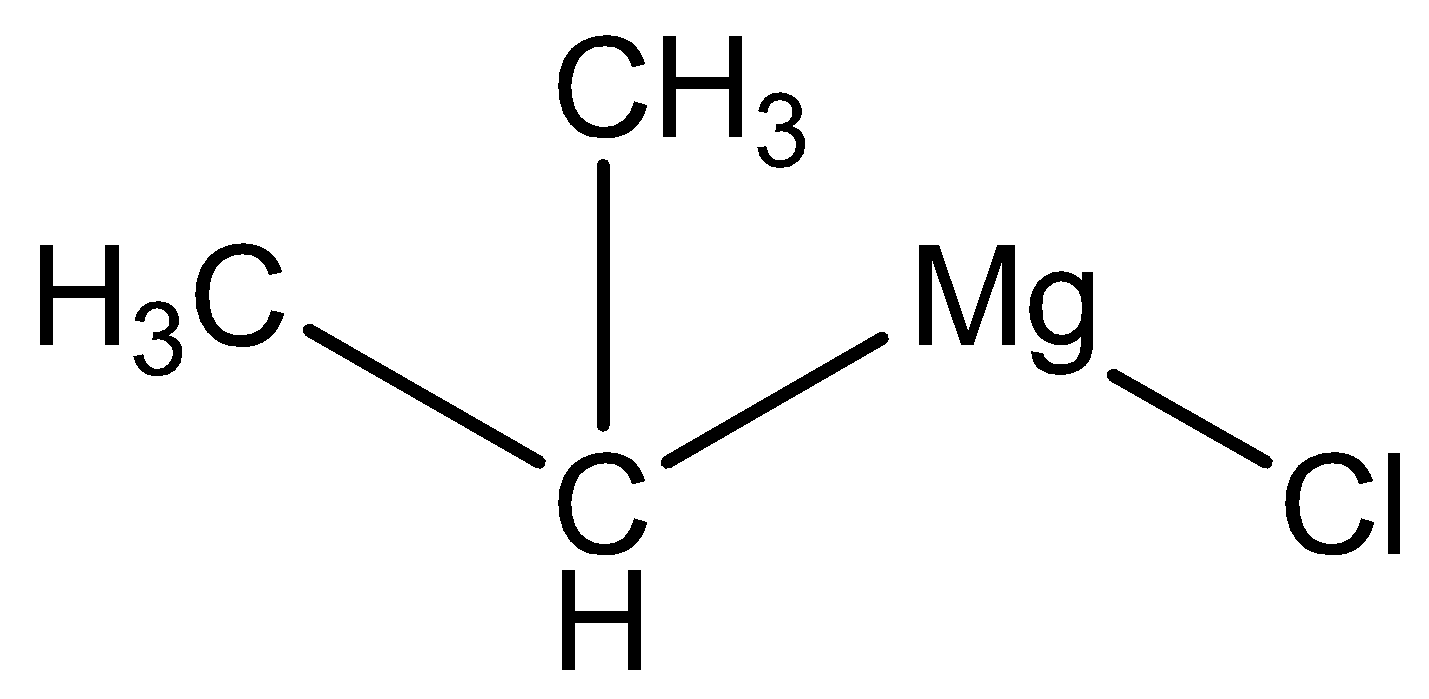
The molecular formula of water is \[{{\text{H}}_{\text{2}}}{\text{O}}\].
The reaction of water on isopropyl magnesium chloride is given below,
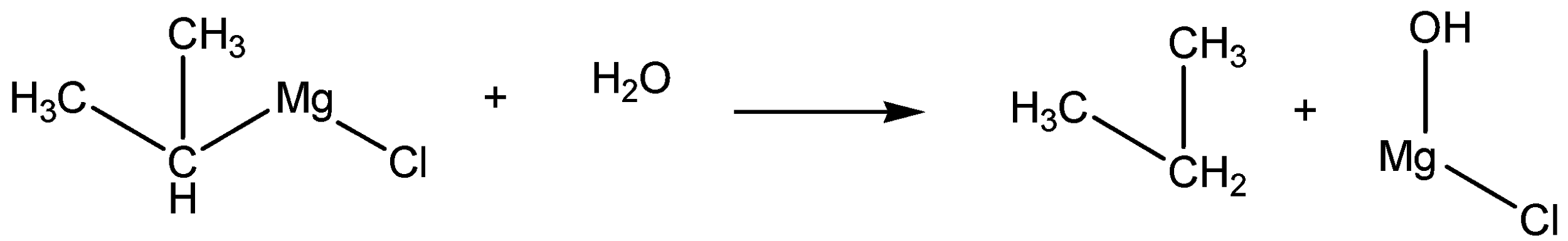
The action of water on isopropyl magnesium chloride is given as n-propane.
The action of water on n-propyl magnesium chloride and isopropyl magnesium chloride is also given as n-propane in both cases.
Note:
We must have to know that the general representation of Grignard reagent is \[{\text{RMgX}}\]. Here R is represent as the alkaline group. It is organic nature in organometallic compound. X is represent as the halogen group. In Grignard mostly used as chlorine, bromine and iodine. Florine is not suitable for Grignard reaction because of the size. Depend on the product and reaction condition we choose\[{\text{RMgCl}}\], \[{\text{RMgBr}}\] and \[{\text{RMgI}}\].
Complete answer:
The given data is
The action of water on n-propyl magnesium chloride to give as,
The molecular formula of n-propyl magnesium chloride is \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - Mg - Cl}}\]
The molecular formula of water is \[{{\text{H}}_{\text{2}}}{\text{O}}\].
The reaction of water on n-propyl magnesium chloride is given below,
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - Mg - Cl + }}{{\text{H}}_{\text{2}}}{\text{O}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{ - C}}{{\text{H}}_{\text{2}}}{\text{ - C}}{{\text{H}}_{\text{3}}} + {\text{HO - Mg - Cl}}\]
The action of water on n-propyl magnesium chloride is given as n-propane.
The action of water on isopropyl magnesium chloride to give as,
The molecular formula of isopropyl magnesium chloride is

The molecular formula of water is \[{{\text{H}}_{\text{2}}}{\text{O}}\].
The reaction of water on isopropyl magnesium chloride is given below,

The action of water on isopropyl magnesium chloride is given as n-propane.
The action of water on n-propyl magnesium chloride and isopropyl magnesium chloride is also given as n-propane in both cases.
Note:
We must have to know that the general representation of Grignard reagent is \[{\text{RMgX}}\]. Here R is represent as the alkaline group. It is organic nature in organometallic compound. X is represent as the halogen group. In Grignard mostly used as chlorine, bromine and iodine. Florine is not suitable for Grignard reaction because of the size. Depend on the product and reaction condition we choose\[{\text{RMgCl}}\], \[{\text{RMgBr}}\] and \[{\text{RMgI}}\].
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




