
(a) What are isomers? Write the structures of two isomers of butane (${{C}_{4}}{{H}_{10}}$).
(b) write the name and chemical equation for the reaction that takes place when \[5%\] solution of alkaline $KMn{{O}_{4}}$ is added drop by drop to warm ethanol.
(c) Name the product formed when ethanol is heated at $443K$ with excess conc. ${{H}_{2}}S{{O}_{4}}$ and the state the role of conc.${{H}_{2}}S{{O}_{4}}$ in the reaction.
Answer
564.6k+ views
Hint: Isomers are those compounds which have similar molecular formula but difference lies in their physical properties. When alkaline $KMn{{O}_{4}}$ is added to ethanol, it forms the compound which consists of the $-COOH$ as the functional group and when ethanol is heated with excess conc.${{H}_{2}}S{{O}_{4}}$, it results in the formation of an unsaturated compound. Now you can easily answer the statements accordingly.
Complete Solution :
We will discuss the statements one by one as;
(a) Isomers are the organic compounds which have the same molecular formula but different properties i.e. structure and the arrangement of the atoms in the space and the phenomenon is known as the isomerism.
The two structural isomers of butane have the same formula as ${{C}_{4}}{{H}_{10}}$ are;
1. n-butane
$C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$
2. Iso-butane
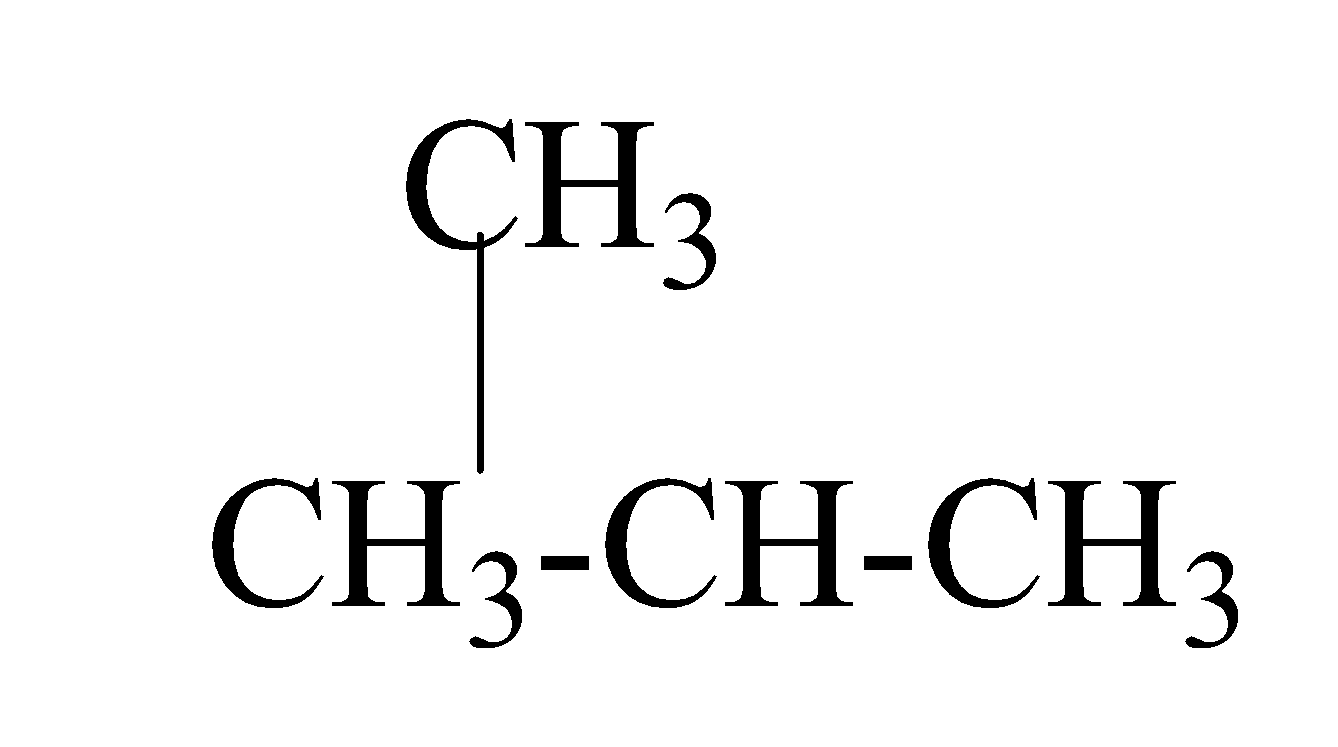
(b) When alkaline $KMn{{O}_{4}}$ is added drop by drop to warm ethanol , it undergoes reduction and results in the formation of acid i.e ethanoic acid. The reaction occurs as;
$C{{H}_{3}}C{{H}_{2}}OH+alk.\text{ }KMn{{O}_{4}}\to C{{H}_{3}}COOH$
(c) When ethanol is heated at $443K$ with excess conc.${{H}_{2}}S{{O}_{4}}$, it results in the formation of alkene along with the water. The reaction occurs as;
${{C}_{2}}{{H}_{5}}OH\xrightarrow[443K]{{{H}_{2}}S{{O}_{4}}}C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}O$
Thus, conc.${{H}_{2}}S{{O}_{4}}$ acts as a dehydrating agent and removes the water from alcohol and forms alkene.
Note: Isomerism are of two types:
1. Structural isomerism :- In this type of isomerism, the molecules have the same molecular formula but differ in the arrangement of the atoms within the molecule. Example : position isomerism, chain isomerism etc.
2. stereoisomerism:- In this type of isomerism, the molecules have the same molecular formula but differ in the arrangement of the atoms in the space. Example: geometrical isomerism, optical isomerism etc.
Complete Solution :
We will discuss the statements one by one as;
(a) Isomers are the organic compounds which have the same molecular formula but different properties i.e. structure and the arrangement of the atoms in the space and the phenomenon is known as the isomerism.
The two structural isomers of butane have the same formula as ${{C}_{4}}{{H}_{10}}$ are;
1. n-butane
$C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$
2. Iso-butane

(b) When alkaline $KMn{{O}_{4}}$ is added drop by drop to warm ethanol , it undergoes reduction and results in the formation of acid i.e ethanoic acid. The reaction occurs as;
$C{{H}_{3}}C{{H}_{2}}OH+alk.\text{ }KMn{{O}_{4}}\to C{{H}_{3}}COOH$
(c) When ethanol is heated at $443K$ with excess conc.${{H}_{2}}S{{O}_{4}}$, it results in the formation of alkene along with the water. The reaction occurs as;
${{C}_{2}}{{H}_{5}}OH\xrightarrow[443K]{{{H}_{2}}S{{O}_{4}}}C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}O$
Thus, conc.${{H}_{2}}S{{O}_{4}}$ acts as a dehydrating agent and removes the water from alcohol and forms alkene.
Note: Isomerism are of two types:
1. Structural isomerism :- In this type of isomerism, the molecules have the same molecular formula but differ in the arrangement of the atoms within the molecule. Example : position isomerism, chain isomerism etc.
2. stereoisomerism:- In this type of isomerism, the molecules have the same molecular formula but differ in the arrangement of the atoms in the space. Example: geometrical isomerism, optical isomerism etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




