
(A) Using Bohr’s second postulate of quantization of orbit angular momentum shows that the circumference of the electron in the nth orbital state in hydrogen atom is n times the de-Broglie wavelength associated with it.
(B) The electron in a hydrogen atom initially in the third excited state. What is the maximum number of spectral lines which can be emitted when it finally moves to the ground state?
Answer
527.3k+ views
Hint: Recall Bohr’s second postulate of quantization which states that, an electron revolves around the nucleus in orbits and the angular momentum of revolution is an integral multiple of $\dfrac{h}{{2p}}$, where $h$ is the planck’s constant. Recall the formula for finding total number of possible spectral lines.
Formula used:
According to Bohr’s second postulate of quantization,
$mv{r_n} = n\dfrac{h}{{2\pi }}$
Where, $m$ is the mass of the electron revolving around the nucleus
$v$ is the velocity of electron in permitted quantized orbit
${r_n}$ is the radius of the orbit
$n$ is the principal quantum number ($n = 1,2,3....$)
$h$ is the planck's constant
Total number of possible spectral line,
$N = \dfrac{{n(n - 1)}}{2}$
Where, $n$ is the value for the excited state
Complete step by step solution:
(A) According to Bohr’s second postulate of quantization,
$mv{r_n} = n\dfrac{h}{{2\pi }}$
On cross multiplication we get,
$ \Rightarrow 2\pi {r_n} = \dfrac{{nh}}{{mv}}$
We know that, $p = mv$
On putting this in the above equation, we get
$ \Rightarrow 2\pi {r_n} = n\dfrac{h}{p}$ ………(i)
Now, according to the de-broglie hypothesis,
$\lambda = \dfrac{h}{p}$ …………(ii)
Where, $\lambda $ is the wavelength associated electron
From equation (i) and (ii) we get
$ \Rightarrow 2\pi {r_n} = n\lambda $
Hence, it is shown that the circumference of the electron in the nth orbital state in hydrogen atom is n times the de-Broglie wavelength associated with it.
(B) It is given in the question that the electron in the hydrogen atom is initially in the third excited state.
As we know that, for ground state $n = 1$
So, for third excited state, $n = 4$
Now, the total number of possible spectral lines is given by the formula,
$N = \dfrac{{n(n - 1)}}{2}$
On putting $n = 4$ in the above equation, we get
$ \Rightarrow N = \dfrac{{4(4 - 1)}}{2}$
On further solving that, we get
$N = 6$
Hence possible transitions are shown in figure below
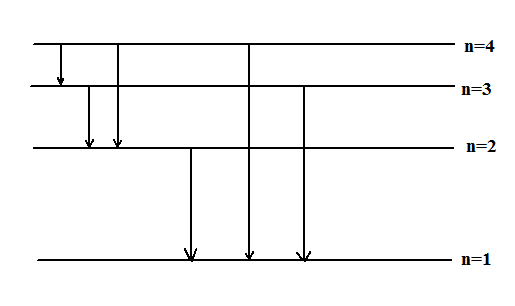
Therefore, the maximum number of spectral lines is 6.
Note: According to the Bohr’s third postulate, an electron can transition from a non-radiating orbit to another of a lower energy level. In doing so, a photon is emitted whose energy is equal to the energy difference between the two states. When the electron is revolving in an orbit closest to the nucleus, the energy of the atom is the least or has the largest negative value.
Formula used:
According to Bohr’s second postulate of quantization,
$mv{r_n} = n\dfrac{h}{{2\pi }}$
Where, $m$ is the mass of the electron revolving around the nucleus
$v$ is the velocity of electron in permitted quantized orbit
${r_n}$ is the radius of the orbit
$n$ is the principal quantum number ($n = 1,2,3....$)
$h$ is the planck's constant
Total number of possible spectral line,
$N = \dfrac{{n(n - 1)}}{2}$
Where, $n$ is the value for the excited state
Complete step by step solution:
(A) According to Bohr’s second postulate of quantization,
$mv{r_n} = n\dfrac{h}{{2\pi }}$
On cross multiplication we get,
$ \Rightarrow 2\pi {r_n} = \dfrac{{nh}}{{mv}}$
We know that, $p = mv$
On putting this in the above equation, we get
$ \Rightarrow 2\pi {r_n} = n\dfrac{h}{p}$ ………(i)
Now, according to the de-broglie hypothesis,
$\lambda = \dfrac{h}{p}$ …………(ii)
Where, $\lambda $ is the wavelength associated electron
From equation (i) and (ii) we get
$ \Rightarrow 2\pi {r_n} = n\lambda $
Hence, it is shown that the circumference of the electron in the nth orbital state in hydrogen atom is n times the de-Broglie wavelength associated with it.
(B) It is given in the question that the electron in the hydrogen atom is initially in the third excited state.
As we know that, for ground state $n = 1$
So, for third excited state, $n = 4$
Now, the total number of possible spectral lines is given by the formula,
$N = \dfrac{{n(n - 1)}}{2}$
On putting $n = 4$ in the above equation, we get
$ \Rightarrow N = \dfrac{{4(4 - 1)}}{2}$
On further solving that, we get
$N = 6$
Hence possible transitions are shown in figure below

Therefore, the maximum number of spectral lines is 6.
Note: According to the Bohr’s third postulate, an electron can transition from a non-radiating orbit to another of a lower energy level. In doing so, a photon is emitted whose energy is equal to the energy difference between the two states. When the electron is revolving in an orbit closest to the nucleus, the energy of the atom is the least or has the largest negative value.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




