
A strong base can abstract an alpha hydrogen from,
(A) ketone
(B) alkane
(C) alkene
(D) amine
Answer
583.8k+ views
Hint: Identify the class of the compounds in which the negative charge obtained on hydrogen abstraction, can be easily delocalized through resonance. This resonance stabilizes this negative charge and facilitates the formation of carbanion.
Complete answer:
Aldehydes and ketones contain carbonyl groups. In the carbonyl group, the carbon-oxygen double bond is present. When a strong base abstracts an alpha hydrogen atom, the alpha carbon atom has a unit negative charge. This negative charge can be easily delocalized to carbonyl oxygen through resonance.
a) Abstraction of $\alpha$ H atom by a base
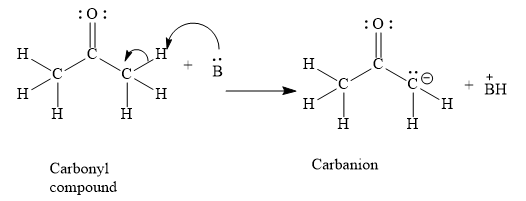
b) Resonance stabilization
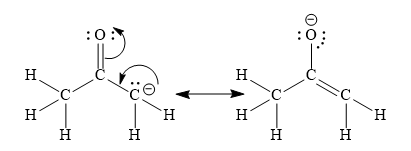
This resonance stabilized the anion. Hence, the formation of this anion is possible.
Hence, a strong base can abstract an alpha hydrogen from a ketone.
Hence the correct option is option (A).
Additional Information: Alkanes, alkenes and amines can also form a carbanion upon extraction of an alpha hydrogen atom by the base. But resonance stabilization of carbanion is not possible for alkanes, alkenes, and amines. Hence, the equilibrium of alkanes (or alkenes or amines) with strong base is much more towards the starting material alkanes (or alkenes or amines). So no appreciable amount of carbanion is obtained.
Note: When a methylene group is present between two carbonyl compounds, it is acidic in nature. The hydrogen atom of this methylene group can be easily abstracted with a suitable base. This principle is used in acetoacetic acid synthesis and malonic acid synthesis.
Complete answer:
Aldehydes and ketones contain carbonyl groups. In the carbonyl group, the carbon-oxygen double bond is present. When a strong base abstracts an alpha hydrogen atom, the alpha carbon atom has a unit negative charge. This negative charge can be easily delocalized to carbonyl oxygen through resonance.
a) Abstraction of $\alpha$ H atom by a base

b) Resonance stabilization

This resonance stabilized the anion. Hence, the formation of this anion is possible.
Hence, a strong base can abstract an alpha hydrogen from a ketone.
Hence the correct option is option (A).
Additional Information: Alkanes, alkenes and amines can also form a carbanion upon extraction of an alpha hydrogen atom by the base. But resonance stabilization of carbanion is not possible for alkanes, alkenes, and amines. Hence, the equilibrium of alkanes (or alkenes or amines) with strong base is much more towards the starting material alkanes (or alkenes or amines). So no appreciable amount of carbanion is obtained.
Note: When a methylene group is present between two carbonyl compounds, it is acidic in nature. The hydrogen atom of this methylene group can be easily abstracted with a suitable base. This principle is used in acetoacetic acid synthesis and malonic acid synthesis.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




