
a solid solution of $\text{ CdB}{{\text{r}}_{\text{2}}}\text{ }$ in $\text{ AgBr }$ contains
A) Schottky defect
B) Frenkel defects
C) Colour centres
D) Frenkel as well as Schottky defect.
Answer
573.3k+ views
Hint: Schottky defect arises when the atoms or ions are missing from their lattice point. This is a vacancy defect that arises when the ionic compound has a high coordination number. Frenkel defect arises when the ion is displaced from their lattice point into the interstitial position. Frenkel defect arises when the ionic compound has low coordination numbers. Silver bromide $\text{ AgBr }$ exists in cubic close packed lattice structure and has a coordination number of 6.
Complete answer:
The Schottky defect arises if some of the lattice points in a crystal are unoccupied. The lattice points in the crystal are unoccupied are called the lattice valencies. There are possibilities of the existence of two vacancies due to cation and one due to anion. The crystal as the whole remains neutral because the number of missing positive and negative ions is the same. The Schottky defect is a vacancy defect which is shown by ionic compounds that have high coordination number and size of cation and anion are near to the same size.
Let's consider a case of silver bromide $\text{ AgBr }$ . Silver bromide has a cubic close packed structure (CCP) lattice. It has a coordination number equal to 6. Coordination number six is not too low nor high. Silver ions are smaller in size and easily get removed from the lattice point. Thus $\text{ AgBr }$exhibits the Schottky defect.
Frenkel defect arises when an ion occupies an interstitial position between the lattice points. Let’s consider a crystal lattice structure of $\text{ AgBr }$as shown below,
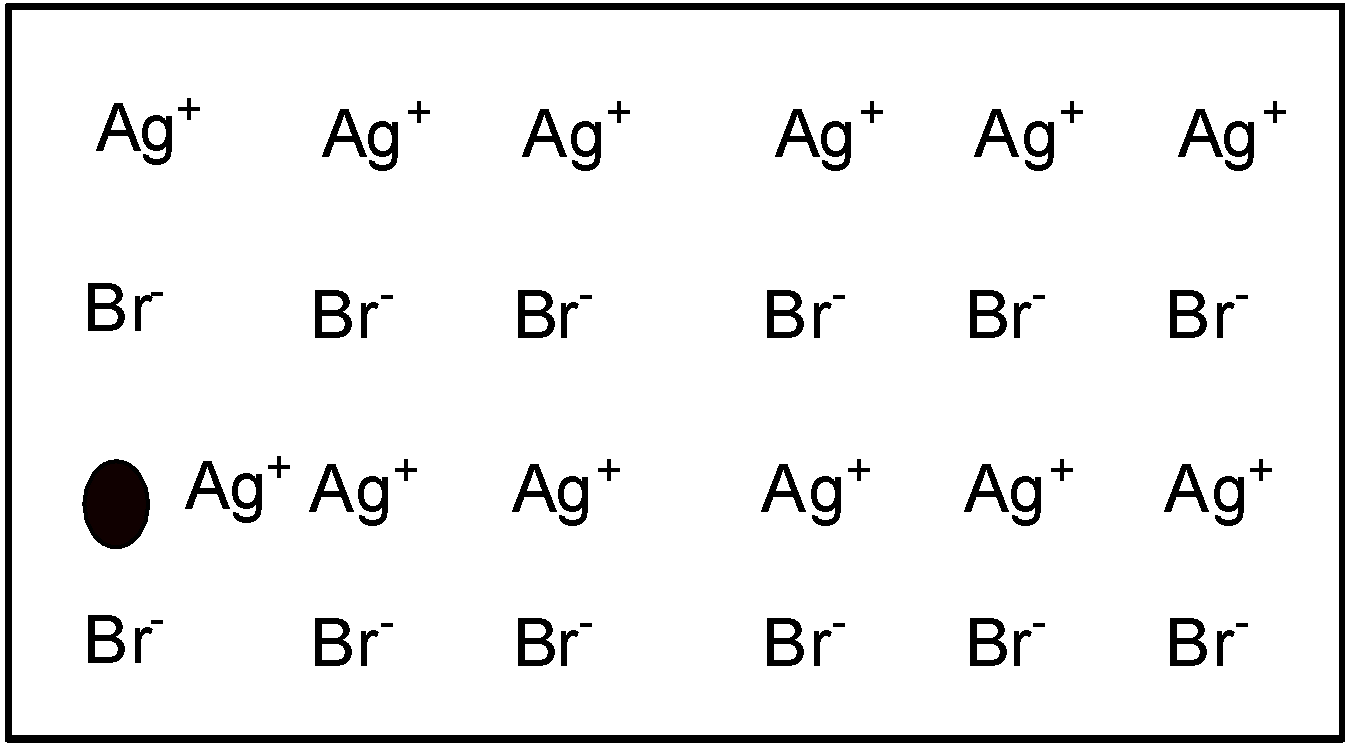
As we can see that one of the $\text{ A}{{\text{g}}^{\text{+}}}\text{ }$ ions occupies a position in the interstitial space rather than its own appropriate site in the lattice. a vacancy is created in the lattice as shown. It may be noted again that the crystal remains neutral since the number of positions of ions is the same as the number of negative ions. Frenkel defect arises in a lattice in which negative ions are much larger than positive ions. It is found in compounds which have a low coordination number. $\text{ AgBr }$ Has a coordination number equal to 6. This coordination number is low. Here in $\text{ AgBr }$the size of the bromide ion is higher than the size of the silver ion. $\text{ Br }$ Occupies octahedral holes and atoms of silver movers to tetrahedral holes. This causes precipitation of cation. thus $\text{ AgBr }$ exhibits a Frenkel defect.
Thus a solid solution $\text{ AgBr }$ contains both Frenkel as well as Schottky defect.
Hence, (D) is the correct option.
Note: Note that, due to the Frenkel defect $\text{ A}{{\text{g}}^{\text{+}}}\text{ }$in the interstitial space of $\text{ AgBr }$ it is responsible for the formation of photographic images on the exposure of $\text{ AgBr }$ crystals to the light. Schottky defect appears generally when the ionic crystal which has positive and negative ions does not differ much in size. However, in $\text{ AgBr }$ only silver ions exhibit Schottky defects.
Complete answer:
The Schottky defect arises if some of the lattice points in a crystal are unoccupied. The lattice points in the crystal are unoccupied are called the lattice valencies. There are possibilities of the existence of two vacancies due to cation and one due to anion. The crystal as the whole remains neutral because the number of missing positive and negative ions is the same. The Schottky defect is a vacancy defect which is shown by ionic compounds that have high coordination number and size of cation and anion are near to the same size.
Let's consider a case of silver bromide $\text{ AgBr }$ . Silver bromide has a cubic close packed structure (CCP) lattice. It has a coordination number equal to 6. Coordination number six is not too low nor high. Silver ions are smaller in size and easily get removed from the lattice point. Thus $\text{ AgBr }$exhibits the Schottky defect.
Frenkel defect arises when an ion occupies an interstitial position between the lattice points. Let’s consider a crystal lattice structure of $\text{ AgBr }$as shown below,

As we can see that one of the $\text{ A}{{\text{g}}^{\text{+}}}\text{ }$ ions occupies a position in the interstitial space rather than its own appropriate site in the lattice. a vacancy is created in the lattice as shown. It may be noted again that the crystal remains neutral since the number of positions of ions is the same as the number of negative ions. Frenkel defect arises in a lattice in which negative ions are much larger than positive ions. It is found in compounds which have a low coordination number. $\text{ AgBr }$ Has a coordination number equal to 6. This coordination number is low. Here in $\text{ AgBr }$the size of the bromide ion is higher than the size of the silver ion. $\text{ Br }$ Occupies octahedral holes and atoms of silver movers to tetrahedral holes. This causes precipitation of cation. thus $\text{ AgBr }$ exhibits a Frenkel defect.
Thus a solid solution $\text{ AgBr }$ contains both Frenkel as well as Schottky defect.
Hence, (D) is the correct option.
Note: Note that, due to the Frenkel defect $\text{ A}{{\text{g}}^{\text{+}}}\text{ }$in the interstitial space of $\text{ AgBr }$ it is responsible for the formation of photographic images on the exposure of $\text{ AgBr }$ crystals to the light. Schottky defect appears generally when the ionic crystal which has positive and negative ions does not differ much in size. However, in $\text{ AgBr }$ only silver ions exhibit Schottky defects.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




