
A primary alcohol, ${C_3}{H_8}O$ (A) on heating with sulphuric acid undergoes dehydration to give an alkene, B . B, when reacted with HCl, gave C, which on treatment with aqueous KOH gives compound D (${C_3}{H_8}O$).
(A) Functional isomers
(B) Position isomers
(C) Chain isomers
(D) stereoisomers
Answer
581.4k+ views
Hint: Firstly, we should know to write the structure of ${C_3}{H_8}O$ and secondly write the reaction following the steps given in the question carefully. Applying Markovnikov rule of addition reaction while adding HCl to alkene obtained may help you in obtaining the correct answer.
Complete step by step answer:
Firstly we will follow the steps given in question,
-Compound A is ${C_3}{H_8}O$, the structure of ${C_3}{H_8}O$:
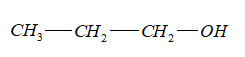
- ${C_3}{H_8}O$ (A) on heating with sulphuric acid undergoes dehydration to give an alkene, B
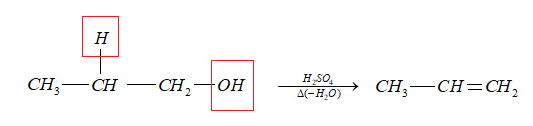
Thus, the alkene (B) is propene.
- When propene reacted with HCl, gave C.
Markovnicov rule states that when R-X is added to an alkene. Between two carbons that share a double bond, the hydrogen will be added to carbon that has a higher number of hydrogen and X will add to carbon that has another substitution group attached to it.
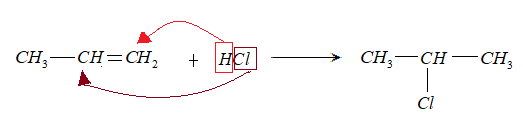
Thus, the compound (C) formed is 2-chloropropane.
- On treatment of 2- chloropropane with aqueous KOH gives compound D (${C_3}{H_8}O$).
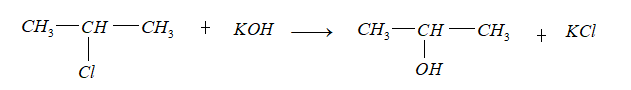
Thus, the compound D formed is 2- propanol.
-If we compare compound A and D, we can come to a conclusion.
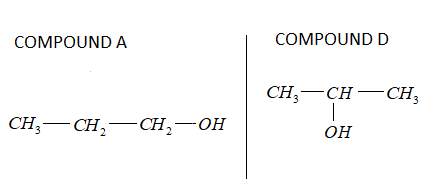
The chemical formula is the same but structured as the position of OH varies in both the compounds.
Thus, option B is the correct answer.
Note: Strictly follow the steps given in the question to arrive at the correct answer. Make sure that in the second step you apply the Markovnikov rule otherwise you will end with the wrong product. If this step goes wrong, the subsequent step too goes wrong.
Complete step by step answer:
Firstly we will follow the steps given in question,
-Compound A is ${C_3}{H_8}O$, the structure of ${C_3}{H_8}O$:
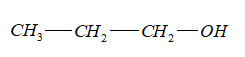
- ${C_3}{H_8}O$ (A) on heating with sulphuric acid undergoes dehydration to give an alkene, B

Thus, the alkene (B) is propene.
- When propene reacted with HCl, gave C.
Markovnicov rule states that when R-X is added to an alkene. Between two carbons that share a double bond, the hydrogen will be added to carbon that has a higher number of hydrogen and X will add to carbon that has another substitution group attached to it.

Thus, the compound (C) formed is 2-chloropropane.
- On treatment of 2- chloropropane with aqueous KOH gives compound D (${C_3}{H_8}O$).
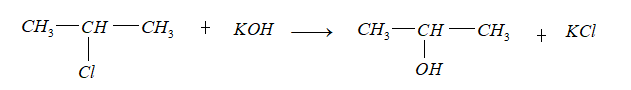
Thus, the compound D formed is 2- propanol.
-If we compare compound A and D, we can come to a conclusion.

The chemical formula is the same but structured as the position of OH varies in both the compounds.
Thus, option B is the correct answer.
Note: Strictly follow the steps given in the question to arrive at the correct answer. Make sure that in the second step you apply the Markovnikov rule otherwise you will end with the wrong product. If this step goes wrong, the subsequent step too goes wrong.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




