
A polymer contains three monomers: acrylonitrile, butadiene and styrene. Predict the structure of this ABS plastic.
Answer
570k+ views
Hint: A monomer refers to the molecule having the capability to form a chemical bond with other molecules present in a long chain. While, a polymer is a complex molecule which generally refers to a chain of several numbers of monomers. In simpler terms, we can say that monomers refer to the building blocks of polymers.
Complete Step by step answer: Acrylonitrile butadiene styrene (ABS) having a chemical formula of \[{\left( {{C_8}{H_8}} \right)_x}.{\left( {{C_4}{H_6}} \right)_y}\cdot{\left( {{C_3}{H_3}N} \right)_z}\]is basically a thermoplastic polymer. ABS plastic is actually a terpolymer which is synthesized by polymerizing acrylonitrile and styrene in the presence of polybutadiene as shown below.
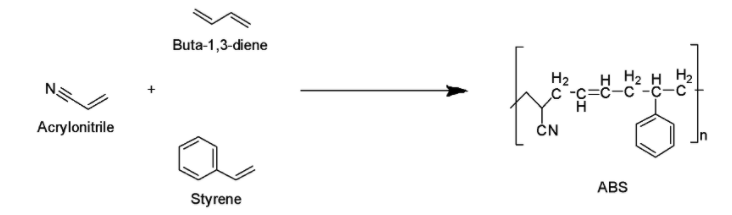
In the resultant product of ABS plastic, a long chain of polybutadiene has been criss-crossed with the shorter chains of poly(styrene-co-acrylonitrile). Actually, the nitrile groups from the neighbouring chains are polar, thus, attract each other and bind the chains together, which as a result, makes ABS stronger compared to pure polystyrene. The acrylonitrile in ABS plastic provides the chemical as well as thermal stability, whereas butadiene provides toughness and strength. On the other hand, styrene provides the polymer a nice and glossy finish.
Note: ABS possess a low melting point, which makes it convenient to use in the injection moulding process as well as 3D printing. ABS is also majorly used in the manufacturing of products such as Drain-Waste-Vent (DWV) pipe systems. Moreover, musical instruments such as clarinets, recorders and plastic oboes, keyboard keycaps and piano movements are also made out of ABS plastic.
Complete Step by step answer: Acrylonitrile butadiene styrene (ABS) having a chemical formula of \[{\left( {{C_8}{H_8}} \right)_x}.{\left( {{C_4}{H_6}} \right)_y}\cdot{\left( {{C_3}{H_3}N} \right)_z}\]is basically a thermoplastic polymer. ABS plastic is actually a terpolymer which is synthesized by polymerizing acrylonitrile and styrene in the presence of polybutadiene as shown below.

In the resultant product of ABS plastic, a long chain of polybutadiene has been criss-crossed with the shorter chains of poly(styrene-co-acrylonitrile). Actually, the nitrile groups from the neighbouring chains are polar, thus, attract each other and bind the chains together, which as a result, makes ABS stronger compared to pure polystyrene. The acrylonitrile in ABS plastic provides the chemical as well as thermal stability, whereas butadiene provides toughness and strength. On the other hand, styrene provides the polymer a nice and glossy finish.
Note: ABS possess a low melting point, which makes it convenient to use in the injection moulding process as well as 3D printing. ABS is also majorly used in the manufacturing of products such as Drain-Waste-Vent (DWV) pipe systems. Moreover, musical instruments such as clarinets, recorders and plastic oboes, keyboard keycaps and piano movements are also made out of ABS plastic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




