
How can a nonpolar molecule contain a polar bond.
Answer
545.4k+ views
Hint: We should know about polar compounds and non-polar compounds. In some triatomic molecules polar bonds are observed in a non-polar compound. The polar bonds are formed by atoms having electronegativity difference by sharing electrons by each atom.
Complete step by step answer:
The covalent bond is formed by the mutual sharing of the electrons between the two atoms to form a molecule.
There are two types of covalent bond, polar covalent bond and non-polar covalent bond.
The polar covalent bond is formed between the two atoms by the mutual sharing of valence electrons by the two atoms. In polar covalent bonds, electronegativity difference is observed between the two atoms. The polar covalent bond between the atoms forms a polar covalent compound.
The non-polar covalent bond is formed between the two atoms by sharing of electrons between the two atoms. In non-polar covalent bond the electronegativity difference is not-observed. Both the atoms have the same electronegativity value. The non-polar covalent bond between the atoms form non-polar covalent compound.
In a non-polar compound like carbon dioxide, polar bonds are observed. It can be explained by first seeing the structure of carbon dioxide. In carbon dioxide, a carbon atom is bonded with oxygen atom by a double bond from one side and on the other side with another oxygen atom by a double bond.
The structure is shown below.
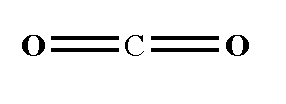
The oxygen atom is more electronegative than the carbon atom, therefore the electron pull will be towards the oxygen atom. Due to electronegativity difference the bond is polar. As on both sides of the carbon atom, two oxygen atoms are present therefore the electronegativity difference is cancelled out, then the carbon dioxide is a non-polar compound.
Note: You should know that the geometry of a carbon dioxide molecule is linear which means that the atoms present in the molecule form a line which have an angle of 180 degree.
Complete step by step answer:
The covalent bond is formed by the mutual sharing of the electrons between the two atoms to form a molecule.
There are two types of covalent bond, polar covalent bond and non-polar covalent bond.
The polar covalent bond is formed between the two atoms by the mutual sharing of valence electrons by the two atoms. In polar covalent bonds, electronegativity difference is observed between the two atoms. The polar covalent bond between the atoms forms a polar covalent compound.
The non-polar covalent bond is formed between the two atoms by sharing of electrons between the two atoms. In non-polar covalent bond the electronegativity difference is not-observed. Both the atoms have the same electronegativity value. The non-polar covalent bond between the atoms form non-polar covalent compound.
In a non-polar compound like carbon dioxide, polar bonds are observed. It can be explained by first seeing the structure of carbon dioxide. In carbon dioxide, a carbon atom is bonded with oxygen atom by a double bond from one side and on the other side with another oxygen atom by a double bond.
The structure is shown below.
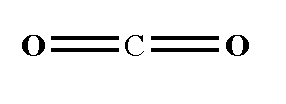
The oxygen atom is more electronegative than the carbon atom, therefore the electron pull will be towards the oxygen atom. Due to electronegativity difference the bond is polar. As on both sides of the carbon atom, two oxygen atoms are present therefore the electronegativity difference is cancelled out, then the carbon dioxide is a non-polar compound.
Note: You should know that the geometry of a carbon dioxide molecule is linear which means that the atoms present in the molecule form a line which have an angle of 180 degree.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




