
A metal complex of coordination number six having three types of ligands a,b and c of composition $M{{a}_{2}}{{b}_{2}}{{c}_{2}}$ can exist in several geometrical isomeric forms; the total number of such isomers is:
(A) 3
(B) 5
(C) 7
(D) 9
Answer
591.9k+ views
Hint: Isomers are compounds having the same molecular formula and have different spatial arrangement. The ligands are arranged in different forms and in cis-trans form. Cis is when same type of ligands are adjacent to each other and trans is when same type are placed opposite to each other.
Complete answer:
A coordination complex consists of a central atom or ion, which is usually metallic and is known as coordination centre, and surrounding bound molecules or ions are known ligands or complexing agents. Many metals containing compounds, especially transition metals, are coordination complexes.
For a coordination compound of coordination number six, that means that the central metal ion is surrounded with six ligands. The shape of a coordination compound with six ligands is known as Octahedral. There are several types of isomers. Geometric Isomer is one of them, having cis-trans and E-Z nomenclature. For, coordination compound having composition $M{{a}_{2}}{{b}_{2}}{{c}_{2}}$ , there will 5 geometric isomers present. All are given as follows.
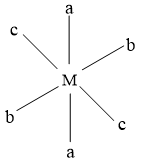
This is a trans isomer, with all the same groups placed opposite to each other.
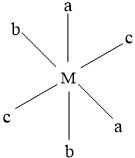
In this isomer, only c type ligands are trans to each other,rest of the two are placed randomly.
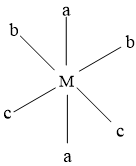
In this isomer, only a type of ligands are trans to each other,rest of the two are placed randomly.
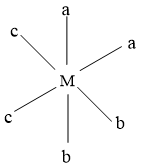
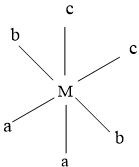
In this isomer, all type of ligands are cis to each other.
Note: E-Z type of isomerism is used when the same type of ligands are not present. The ligands are ranked as per their priority rule and when two same priority groups are placed on the same side then it is named as Z and when same priority groups are placed opposite side, then it is named E.
Complete answer:
A coordination complex consists of a central atom or ion, which is usually metallic and is known as coordination centre, and surrounding bound molecules or ions are known ligands or complexing agents. Many metals containing compounds, especially transition metals, are coordination complexes.
For a coordination compound of coordination number six, that means that the central metal ion is surrounded with six ligands. The shape of a coordination compound with six ligands is known as Octahedral. There are several types of isomers. Geometric Isomer is one of them, having cis-trans and E-Z nomenclature. For, coordination compound having composition $M{{a}_{2}}{{b}_{2}}{{c}_{2}}$ , there will 5 geometric isomers present. All are given as follows.

This is a trans isomer, with all the same groups placed opposite to each other.

In this isomer, only c type ligands are trans to each other,rest of the two are placed randomly.

In this isomer, only a type of ligands are trans to each other,rest of the two are placed randomly.

In this isomer, all type of ligands are cis to each other.

In this iomer, b types ligand is trans to each other, and a and c type ligand are cis to each other.
So, the correct answer is “Option B”.
Note: E-Z type of isomerism is used when the same type of ligands are not present. The ligands are ranked as per their priority rule and when two same priority groups are placed on the same side then it is named as Z and when same priority groups are placed opposite side, then it is named E.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




