
A lead ball at ${30^0}C$ is dropped from a height of 6.2 km. The ball is heated due to the air resistance and it completely melts just before reaching the ground. The molten substance falls slowly on the ground. If the specific heat of lead = $126JK{g^{ - 1}}$ $^0{C^{ - 1}}$and melting point of lead = ${130^0}C$ and suppose that any mechanical energy lost is used to heat the ball, then the latent heat of fusion of lead is
A) $2.4 \times {10^4}JK{g^{ - 1}}$
B) $3.6 \times {10^4}JK{g^{ - 1}}$
C) $7.6 \times {10^2}JK{g^{ - 1}}$
D) $4.9 \times {10^4}JK{g^{ - 1}}$
E) $7.9 \times {10^4}JK{g^{ - 1}}$
Answer
589.2k+ views
Hint:The lead ball has some heat energy when reaching the melting point and different heat energy when it melts completely. We will find out the relation of before and after heat energy of the ball and then it is given that mechanical energy is lost to heat the ball then mgh=net heat of the ball. This will give the latent heat of fusion of the lead ball, where m is mass, g is gravity, and height of the ball.
Complete step by step answer:
Step 1:
Latent heat of fusion :- Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically in the form of heat) in order to trigger a change in its physical state and convert it into a liquid (when the pressure of the environment is kept constant).
We are given:
A lead ball at ${30^0}C$ is dropped from a height of 6.2 km
The specific heat of lead = $126JK{g^{ - 1}}$ $^0{C^{ - 1}}$
The point of lead = ${130^0}C$
We need to find the latent heat of fusion of lead
Let us take the help of the diagram to understand the question:
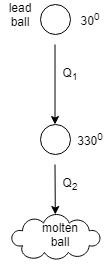
The ball when at rest has the temperature ${30^0}C$ and then when it is released it must have attained the temperature of ${330^0}C$ and reached the ground in the molten state. Then there must be heat energy ${Q_1}$ before reaching melting temperature and heat energy ${Q_2}$ at the time of melting. The rise in temperature is ${300^0}C$
Step 2:
Now coming to the question:
We are not given exactly the mass of the lead ball
Then let the mass of the ball be m so, ${Q_1}$=$m \times 126 \times 300$ here, m is the mass of the ball
In the same way the ${Q_2}$ =m × L where, L is the latent heat of fusion of lead
To find ${Q_{NET}}$ we need to add ${Q_1}$ and ${Q_2}$
Adding both we get, ${Q_{NET}}$=$m\left( {126 \times 300 \times L} \right)$
We are given that any mechanical energy lost is used to heat the ball then; loss in mechanical energy is equal to heat.
Then, $mgh = {m_s}\Delta \theta + mL$ here, g is the gravity, h is the height
Taking left hand side mgh and equating to the ${Q_{NET}}$
This will equal to $m\left( {126 \times 300 + L} \right) = m \times 10 \times 6.2 \times {10^3}$
Here, mass will get cancel out and from there we will get L equal to $2.4 \times {10^4}JK{g^{ - 1}}$
Hence, the latent heat of fusion of lead is $2.4 \times {10^4}JK{g^{ - 1}}$
Option A is correct.
Note:Reason why we choose latent heat of fusion: The latent heat of fusion of a substance also accounts for the energy required to accommodate any increase in the volume of the substance post the change of its physical state. The temperature at which the substance undergoes the phase transition is called the melting point of the substance.
Complete step by step answer:
Step 1:
Latent heat of fusion :- Latent heat of fusion, also known as enthalpy of fusion, is the amount of energy that must be supplied to a solid substance (typically in the form of heat) in order to trigger a change in its physical state and convert it into a liquid (when the pressure of the environment is kept constant).
We are given:
A lead ball at ${30^0}C$ is dropped from a height of 6.2 km
The specific heat of lead = $126JK{g^{ - 1}}$ $^0{C^{ - 1}}$
The point of lead = ${130^0}C$
We need to find the latent heat of fusion of lead
Let us take the help of the diagram to understand the question:

The ball when at rest has the temperature ${30^0}C$ and then when it is released it must have attained the temperature of ${330^0}C$ and reached the ground in the molten state. Then there must be heat energy ${Q_1}$ before reaching melting temperature and heat energy ${Q_2}$ at the time of melting. The rise in temperature is ${300^0}C$
Step 2:
Now coming to the question:
We are not given exactly the mass of the lead ball
Then let the mass of the ball be m so, ${Q_1}$=$m \times 126 \times 300$ here, m is the mass of the ball
In the same way the ${Q_2}$ =m × L where, L is the latent heat of fusion of lead
To find ${Q_{NET}}$ we need to add ${Q_1}$ and ${Q_2}$
Adding both we get, ${Q_{NET}}$=$m\left( {126 \times 300 \times L} \right)$
We are given that any mechanical energy lost is used to heat the ball then; loss in mechanical energy is equal to heat.
Then, $mgh = {m_s}\Delta \theta + mL$ here, g is the gravity, h is the height
Taking left hand side mgh and equating to the ${Q_{NET}}$
This will equal to $m\left( {126 \times 300 + L} \right) = m \times 10 \times 6.2 \times {10^3}$
Here, mass will get cancel out and from there we will get L equal to $2.4 \times {10^4}JK{g^{ - 1}}$
Hence, the latent heat of fusion of lead is $2.4 \times {10^4}JK{g^{ - 1}}$
Option A is correct.
Note:Reason why we choose latent heat of fusion: The latent heat of fusion of a substance also accounts for the energy required to accommodate any increase in the volume of the substance post the change of its physical state. The temperature at which the substance undergoes the phase transition is called the melting point of the substance.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




