
A hydrocarbon containing $2$ carbon atoms gives Sabatier and Senderens reaction but does not participate with ammoniacal silver nitrate solution. The hydrocarbon in question is
A. Ethane
B. Acetylene
C. Ethylene
D. None of these
Answer
361.5k+ views
Hint: A hydrocarbon containing two carbon atoms is generally alkane, alkene, or alkyne. Only alkenes and alkynes undergo Sabatier and senderens reactions. Among these only alkynes precipitate with ammoniacal silver nitrate solution. To solve this question we have to find out the alkenes.
Complete Step by Step Answer:
A hydrocarbon containing two carbon atoms is an alkane, alkene, and alkyne having general formula ${{C}_{n}}{{H}_{2n+2}}$,${{C}_{n}}{{H}_{2n}}$, and ${{C}_{n}}{{H}_{2n-2}}$respectively (where n is number of carbon atoms). Alkenes and alkynes react with molecular hydrogen in presence of Raney $Ni$within the temperature range ${{250}^{{\mathrm O}}}-{{300}^{O}}C$ are known as Sabatier and Senderens reaction to produce saturated alkane compounds.
Alkanes and alkenes do not precipitate with ammoniacal silver nitrate solution but alkynes do participate. Generally, aliphatic and aromatic aldehydes give Tollens test with ammoniacal silver nitrate solutions. Terminal alkynes react with tollen’s reagent to give white precipitates.
Here we have three hydrocarbons containing two carbon atoms. In (A) Ethane is an alkane compound having chemical formula ${{C}_{2}}{{H}_{6}}$. This hydrocarbon neither participates in Sabatier and Senderens reaction nor reacts with tollen’s reagent. Hence it is the wrong option.
Next, we have an alkyne compound, acetylene having chemical formula ${{C}_{2}}{{H}_{2}}$ . Acetylene participates in Sabatier and Senderens reaction and also gives a precipitate with tollen’s reagent.
In the third option ethylene, an alkene compound having chemical formula ${{C}_{2}}{{H}_{4}}$ gives Sabatier and Senderens reaction to form ethane and does not precipitate with ammoniacal silver nitrate solution.
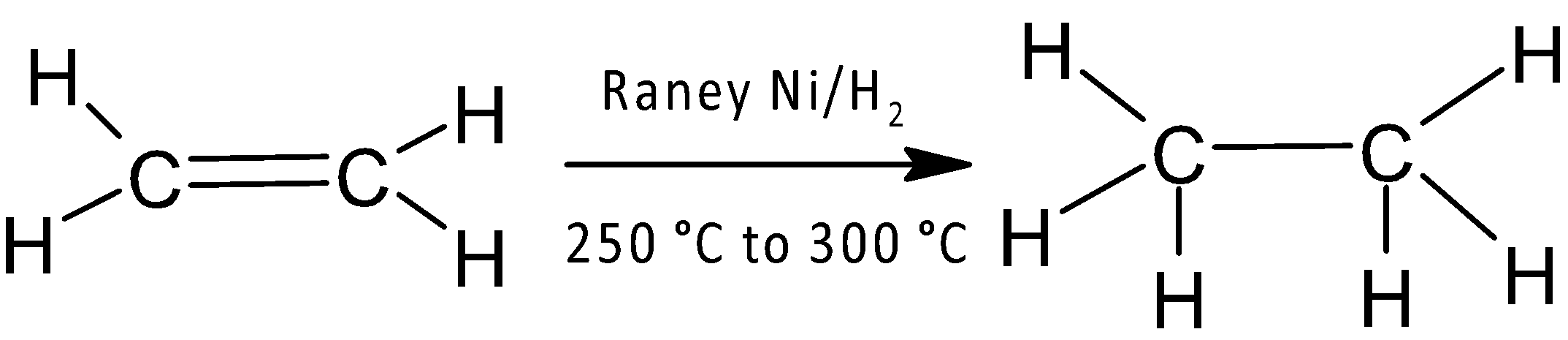
Thus, option (C) is correct.
Note: Tollen’s reagent is prepared by mixing silver nitrate with ammonium hydroxide solution. It is a mild oxidising agent and can selectively oxidise carbonyl compounds that are aldehyde. This reagent is specially used to distinguish between aldehydes and ketones. Here aldehydes do react with ammoniacal silver nitrate to give carboxylic acid but ketones do not react with tollen’s reagent
Complete Step by Step Answer:
A hydrocarbon containing two carbon atoms is an alkane, alkene, and alkyne having general formula ${{C}_{n}}{{H}_{2n+2}}$,${{C}_{n}}{{H}_{2n}}$, and ${{C}_{n}}{{H}_{2n-2}}$respectively (where n is number of carbon atoms). Alkenes and alkynes react with molecular hydrogen in presence of Raney $Ni$within the temperature range ${{250}^{{\mathrm O}}}-{{300}^{O}}C$ are known as Sabatier and Senderens reaction to produce saturated alkane compounds.
Alkanes and alkenes do not precipitate with ammoniacal silver nitrate solution but alkynes do participate. Generally, aliphatic and aromatic aldehydes give Tollens test with ammoniacal silver nitrate solutions. Terminal alkynes react with tollen’s reagent to give white precipitates.
Here we have three hydrocarbons containing two carbon atoms. In (A) Ethane is an alkane compound having chemical formula ${{C}_{2}}{{H}_{6}}$. This hydrocarbon neither participates in Sabatier and Senderens reaction nor reacts with tollen’s reagent. Hence it is the wrong option.
Next, we have an alkyne compound, acetylene having chemical formula ${{C}_{2}}{{H}_{2}}$ . Acetylene participates in Sabatier and Senderens reaction and also gives a precipitate with tollen’s reagent.
In the third option ethylene, an alkene compound having chemical formula ${{C}_{2}}{{H}_{4}}$ gives Sabatier and Senderens reaction to form ethane and does not precipitate with ammoniacal silver nitrate solution.

Thus, option (C) is correct.
Note: Tollen’s reagent is prepared by mixing silver nitrate with ammonium hydroxide solution. It is a mild oxidising agent and can selectively oxidise carbonyl compounds that are aldehyde. This reagent is specially used to distinguish between aldehydes and ketones. Here aldehydes do react with ammoniacal silver nitrate to give carboxylic acid but ketones do not react with tollen’s reagent
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

What is the median of the first 10 natural numbers class 10 maths CBSE




