
(a)- How will you convert the following:
(i)- Propanone to Propan- 2 –ol
(ii)- Ethanal to 2- hydroxyl propanoic acid
(iii)- Toluene to benzoic acid
(b)- Given simple chemical test to distinguish between:
(i)- Pentan- 2 –one and Pentan- 3 –one
(ii)- Ethanal and Propanal
Answer
542.4k+ views
Hint:To convert a ketone to alcohol, we have to reduce the ketone. When the aldehyde is treated with hydrogen cyanide and then it is hydrolyzed to get hydroxyl carboxylic acid. Toluene is a hydrocarbon, so when it is oxidized we can get the benzoic acid. Iodoform test is a test that can be used to differentiate many compounds.
Complete step-by-step answer:(a)- (i)- Propanone to Propan- 2 –ol
Propanone is a compound in which the functional group is ketone, so when it is reduced with sodium in the presence of alcohol, it is converted into Propan- 2 –ol. The reaction is given below:
$C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}\xrightarrow{Na/alcohol}C{{H}_{3}}-\overset{OH}{\mathop{\overset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}$
(ii)- Ethanal to 2- hydroxyl propanoic acid
Ethanal is a compound in which the functional group is an aldehyde. When it is treated with hydrogen cyanide then the cyanide group will attach with the carbon atom and the hydrogen atom will attach with an oxygen atom. This compound on hydrolysis will give 2- hydroxyl propanoic acid. The reaction is given below:
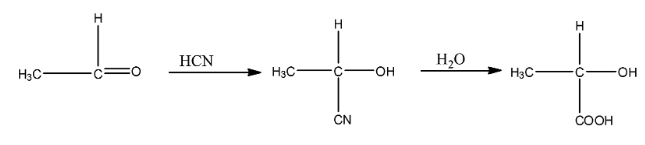
(iii)- Toluene to benzoic acid
Toluene is an aromatic hydrocarbon, in which a methyl group is present on the benzene ring, when it is treated with a strong oxidizing agent like potassium dichromate in the presence of sulfuric acid will give benzoic acid. The reaction is given below:
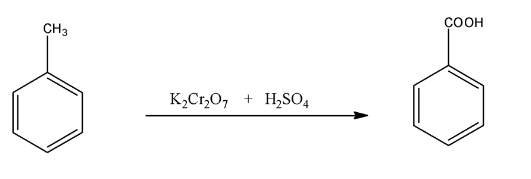
(b)- (i)- Pentan- 2 –one and Pentan- 3 –one
In pentan-2-one there is a methyl ketone group that can give the Iodoform test. But in pentan-3-one there is no methyl ketone group present, so it will not give the Iodoform test. The reaction of pentan-2-one is given below:
$C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C(O)-C{{H}_{3}}+3NaOI\to C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-COONa+CH{{I}_{3}}$
The Iodoform formed in this will give the ppt.
(ii)- Ethanal and Propanal
In ethanal, there is a methyl ketone group that can give the Iodoform test. But, in Propanal there is no methyl ketone group present, so it will not give the Iodoform test. The reaction of ethanal is given below:
$C{{H}_{3}}-C(O)-H+NaOI\to CHOONa+CH{{I}_{3}}$
The Iodoform formed in this will give the ppt.
Note:When any aromatic compound having a substituent carbon atom having a hydrogen atom on oxidation will give benzoic acid. When the cyanide group is hydrolyzed, there is the formation of acid and when the amide group is hydrolyzed, then there is the formation of acid.
Complete step-by-step answer:(a)- (i)- Propanone to Propan- 2 –ol
Propanone is a compound in which the functional group is ketone, so when it is reduced with sodium in the presence of alcohol, it is converted into Propan- 2 –ol. The reaction is given below:
$C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}\xrightarrow{Na/alcohol}C{{H}_{3}}-\overset{OH}{\mathop{\overset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}$
(ii)- Ethanal to 2- hydroxyl propanoic acid
Ethanal is a compound in which the functional group is an aldehyde. When it is treated with hydrogen cyanide then the cyanide group will attach with the carbon atom and the hydrogen atom will attach with an oxygen atom. This compound on hydrolysis will give 2- hydroxyl propanoic acid. The reaction is given below:

(iii)- Toluene to benzoic acid
Toluene is an aromatic hydrocarbon, in which a methyl group is present on the benzene ring, when it is treated with a strong oxidizing agent like potassium dichromate in the presence of sulfuric acid will give benzoic acid. The reaction is given below:

(b)- (i)- Pentan- 2 –one and Pentan- 3 –one
In pentan-2-one there is a methyl ketone group that can give the Iodoform test. But in pentan-3-one there is no methyl ketone group present, so it will not give the Iodoform test. The reaction of pentan-2-one is given below:
$C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C(O)-C{{H}_{3}}+3NaOI\to C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-COONa+CH{{I}_{3}}$
The Iodoform formed in this will give the ppt.
(ii)- Ethanal and Propanal
In ethanal, there is a methyl ketone group that can give the Iodoform test. But, in Propanal there is no methyl ketone group present, so it will not give the Iodoform test. The reaction of ethanal is given below:
$C{{H}_{3}}-C(O)-H+NaOI\to CHOONa+CH{{I}_{3}}$
The Iodoform formed in this will give the ppt.
Note:When any aromatic compound having a substituent carbon atom having a hydrogen atom on oxidation will give benzoic acid. When the cyanide group is hydrolyzed, there is the formation of acid and when the amide group is hydrolyzed, then there is the formation of acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




