
a) Explain the preparation of Nylon-6,6 with equation.
b) What are thermoplastic polymers? Give an example.
c) Write the structure of isoprene (2-methyl-1,3-butadiene).
Answer
595.2k+ views
Hint: Think about what the names of the polymers Nylon-6,6 and 2-methyl-1,3-butadiene may mean. Try to figure out the length of the carbon chain or the location of the functional groups on the chain based on that.
For the second question, break down the word thermoplastic and think about how heat may cause the polymer to behave like a plastic material.
Complete step by step solution:
a) First of all, we have to know what Nylon-6,6 is. Nylon-6,6 is a polymer used in textile and plastics industries. Nylon-6,6 is made of two monomers, they are hexamethylenediamine and adipic acid.
Nylon-6,6 is prepared by polycondensation of hexamethylenediamine and adipic acid. Equivalent amounts of hexamethylenediamine and adipic acid are combined with water in a reactor. This is crystallized to make nylon salt, an ammonium-carboxylate mixture. The nylon salt goes into a reaction vessel where the polymerization process takes place either in batches or continuously. Let us look the reaction:
\[\begin{align}
& n[HOOC-{{(C{{H}_{2}})}_{4}}-COOH]+n[{{H}_{2}}N-{{(C{{H}_{2}})}_{6}}-N{{H}_{2}}]\to \\
& {{\left[ -OC-(C{{H}_{2}})-CO-NH-{{(C{{H}_{2}})}_{6}}-NH- \right]}_{n}}+(2n-1){{H}_{2}}O \\
\end{align}\]
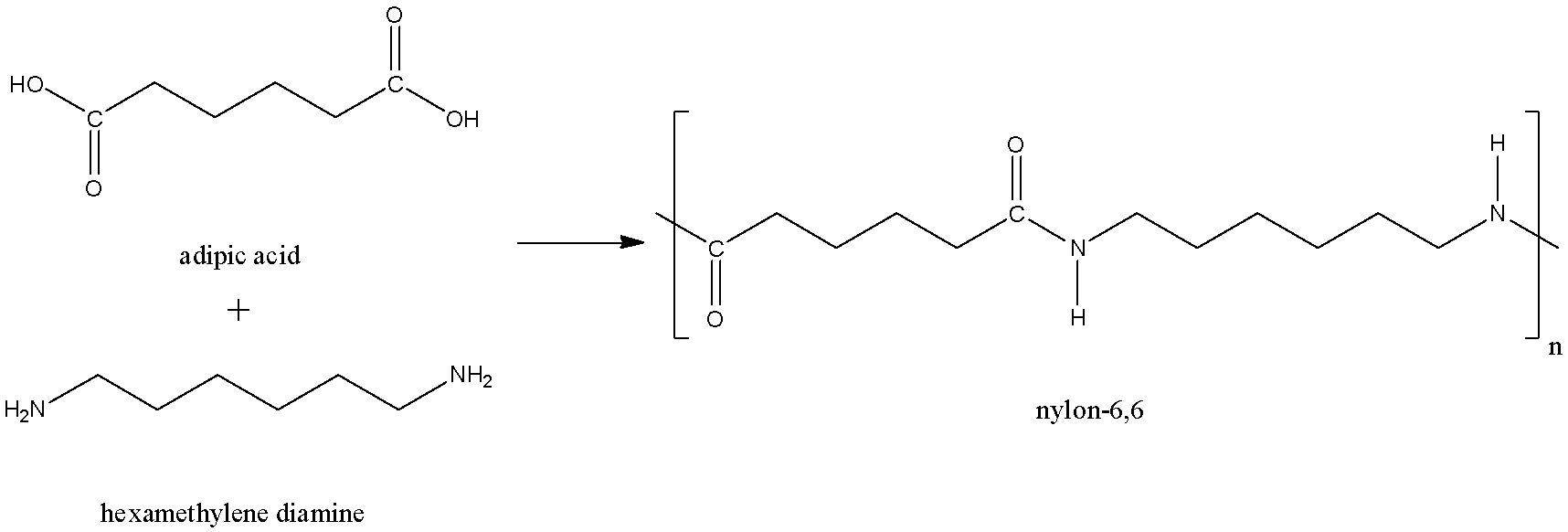
Removing water drives the reaction toward polymerization through the formation of amide bonds from the acid and amine functions. Thus, molten Nylon-6,6 is formed.
b) Thermoplastic polymers are all the plastic materials which can be softened and melted by heating but they set again when cool. When heated, thermoplastic polymers are easy to form into a variety of shapes, thermoplastic polymers soften and also lend themselves to recycling.
Examples for thermoplastic polymers include polyethylene, polypropylene, polyvinyl chloride, polystyrene, Nylon, Teflon, etc.
c) We know that the isoprene is 2-methyl-1,3-butadiene. We can deduce that the parent carbon chain has 4 carbon atoms. There is a methyl substituent at carbon number 2. Carbon number 1 and 3 have a double bond. From this we can say that the structure of isoprene is:
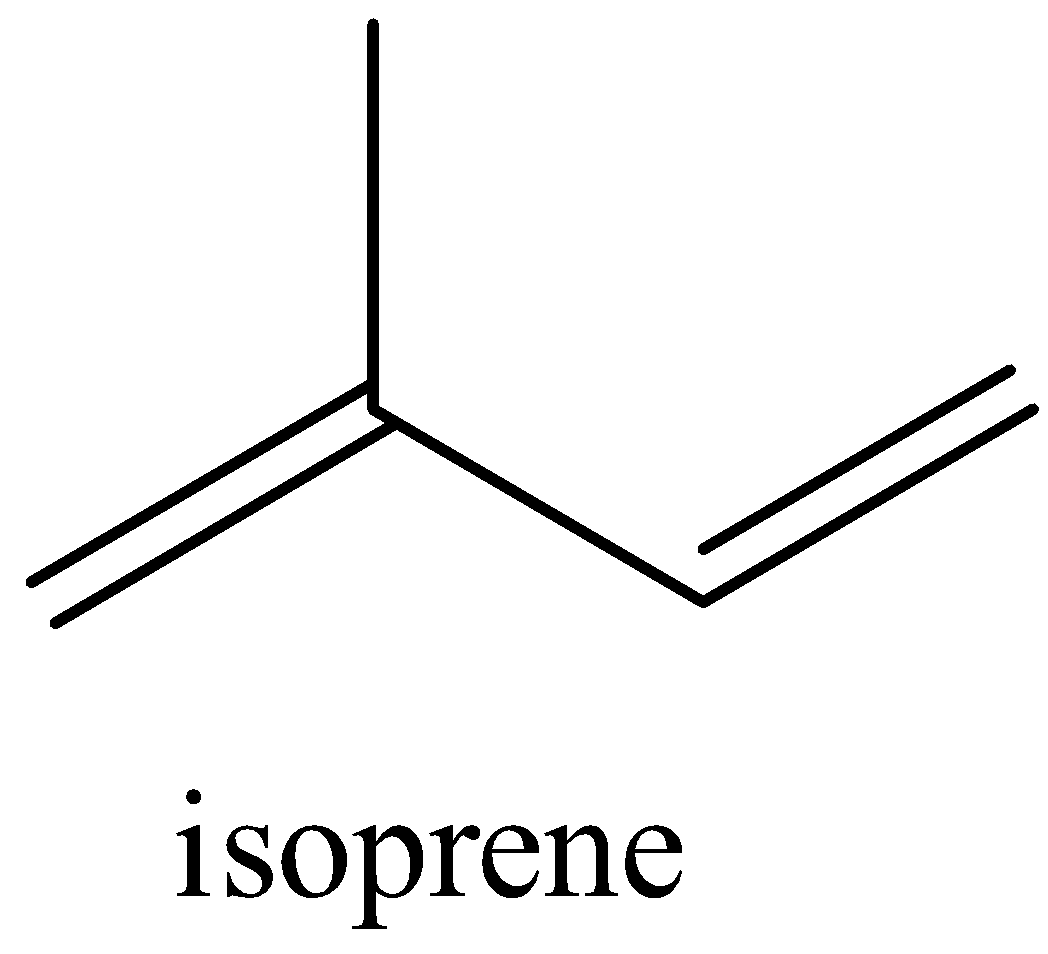
Note: The two monomers of Nylon-6,6 are hexamethylenediamine and adipic acids both contain 6 numbers of carbon atom, which gives nylon-6,6 its name.
Plastic is a word derived from one of its properties. A plastic material is a material that does not go back to its original shape when stress is applied to it. Hence, when thermoplastic polymers are heated and stress is applied to them for moulding, they do not go back to their original shape.
For the second question, break down the word thermoplastic and think about how heat may cause the polymer to behave like a plastic material.
Complete step by step solution:
a) First of all, we have to know what Nylon-6,6 is. Nylon-6,6 is a polymer used in textile and plastics industries. Nylon-6,6 is made of two monomers, they are hexamethylenediamine and adipic acid.
Nylon-6,6 is prepared by polycondensation of hexamethylenediamine and adipic acid. Equivalent amounts of hexamethylenediamine and adipic acid are combined with water in a reactor. This is crystallized to make nylon salt, an ammonium-carboxylate mixture. The nylon salt goes into a reaction vessel where the polymerization process takes place either in batches or continuously. Let us look the reaction:
\[\begin{align}
& n[HOOC-{{(C{{H}_{2}})}_{4}}-COOH]+n[{{H}_{2}}N-{{(C{{H}_{2}})}_{6}}-N{{H}_{2}}]\to \\
& {{\left[ -OC-(C{{H}_{2}})-CO-NH-{{(C{{H}_{2}})}_{6}}-NH- \right]}_{n}}+(2n-1){{H}_{2}}O \\
\end{align}\]

Removing water drives the reaction toward polymerization through the formation of amide bonds from the acid and amine functions. Thus, molten Nylon-6,6 is formed.
b) Thermoplastic polymers are all the plastic materials which can be softened and melted by heating but they set again when cool. When heated, thermoplastic polymers are easy to form into a variety of shapes, thermoplastic polymers soften and also lend themselves to recycling.
Examples for thermoplastic polymers include polyethylene, polypropylene, polyvinyl chloride, polystyrene, Nylon, Teflon, etc.
c) We know that the isoprene is 2-methyl-1,3-butadiene. We can deduce that the parent carbon chain has 4 carbon atoms. There is a methyl substituent at carbon number 2. Carbon number 1 and 3 have a double bond. From this we can say that the structure of isoprene is:

Note: The two monomers of Nylon-6,6 are hexamethylenediamine and adipic acids both contain 6 numbers of carbon atom, which gives nylon-6,6 its name.
Plastic is a word derived from one of its properties. A plastic material is a material that does not go back to its original shape when stress is applied to it. Hence, when thermoplastic polymers are heated and stress is applied to them for moulding, they do not go back to their original shape.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




