
(a) Draw the structures of the following molecules:
\[
{\left( i \right){\text{ }}XeO{F_4}} \\
{\left( {ii} \right){\text{ }}{H_2}S{O_4}}
\]
(b) Write the structural difference between white phosphorus and red phosphorus.
Answer
577.5k+ views
Hint: Lewis structures or Lewis dot diagrams represent the structures that depict the bonding between the atoms of a molecule as well as the lone pair of electrons which may exist in a molecule.
Complete solution
a. In order to draw the Lewis dot structure, you must calculate the number of valence shell electrons in the compound. Let us draw the structure of each molecule one by one.
\[\left( i \right){\text{ }}XeO{F_4}\]
Number of valence shell electrons in \[XeO{F_4}\] can be calculated as follows:
\[
{ = Xe + O + 4\left( F \right)} \\
{ = 8 + 6 + 4\left( 7 \right)} \\
{ = 14 + 28} \\
{ = 42}
\]
Therefore \[XeO{F_4}\] has 42 valence shell electrons. These valence electrons are allotted to the elements such that each element attains octet. But in the present case, Xenon is exceptional since it can expand its octet. Thus, the structure of \[XeO{F_4}\] is shown below:
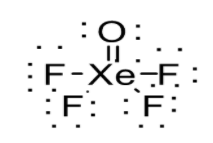
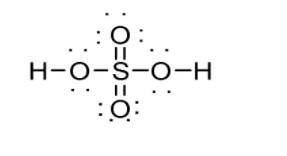
\[\left( {ii} \right){\text{ }}{{\text{H}}_2}S{O_4}\]
Number of valence shell electrons in\[{{\text{H}}_2}S{O_4}\] can be calculated as follows:
\[
{ = 2(H) + S + 4\left( O \right)} \\
{ = 2(1) + 6 + 4\left( 6 \right)} \\
{ = 2 + 6 + 24} \\
{ = 32}
\]
Therefore \[{{\text{H}}_2}S{O_4}\] has 32 valence shell electrons. If you divide this by 2 i.e. $\dfrac{{32}}{2} = 16$ number of electron pairs which are either shared by elements via bonds or lone pairs of electrons in the Lewis Structure. In the structure, line as well as pair dots denote the electron pair. If you count these both (line and pair of dots), you would see 16. Hydrogen can possess a maximum of 2 electrons, whereas other elements can possess a maximum of 8. Hydrogen atoms can be attached to the outside of oxygen molecules. The structure of \[{{\text{H}}_2}S{O_4}\] is shown below:
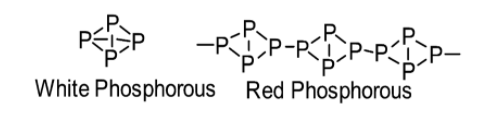
(b) The structural difference between white phosphorus and red phosphorus is stated below: White phosphorus consists of \[{P_4}\] molecules. Thus, white phosphorus exists as a \[{P_4}\] molecule in both solid as well as vapour states. On the other hand, the crystal structure of red phosphorus possesses a complicated network of bonding (it is polymeric) as it occurs as a chain of tetrahedral \[{P_4}\] units.
Note:
The main purpose to draw a Lewis dot structure is to determine the lone pair of electrons in molecules which helps to identify the formation of chemical bonds. Lewis structures can be drawn for molecules containing the covalent bonds as well as for coordination compounds.
Complete solution
a. In order to draw the Lewis dot structure, you must calculate the number of valence shell electrons in the compound. Let us draw the structure of each molecule one by one.
\[\left( i \right){\text{ }}XeO{F_4}\]
Number of valence shell electrons in \[XeO{F_4}\] can be calculated as follows:
\[
{ = Xe + O + 4\left( F \right)} \\
{ = 8 + 6 + 4\left( 7 \right)} \\
{ = 14 + 28} \\
{ = 42}
\]
Therefore \[XeO{F_4}\] has 42 valence shell electrons. These valence electrons are allotted to the elements such that each element attains octet. But in the present case, Xenon is exceptional since it can expand its octet. Thus, the structure of \[XeO{F_4}\] is shown below:
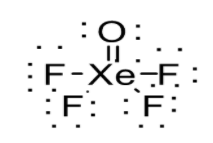
\[\left( {ii} \right){\text{ }}{{\text{H}}_2}S{O_4}\]
Number of valence shell electrons in\[{{\text{H}}_2}S{O_4}\] can be calculated as follows:
\[
{ = 2(H) + S + 4\left( O \right)} \\
{ = 2(1) + 6 + 4\left( 6 \right)} \\
{ = 2 + 6 + 24} \\
{ = 32}
\]
Therefore \[{{\text{H}}_2}S{O_4}\] has 32 valence shell electrons. If you divide this by 2 i.e. $\dfrac{{32}}{2} = 16$ number of electron pairs which are either shared by elements via bonds or lone pairs of electrons in the Lewis Structure. In the structure, line as well as pair dots denote the electron pair. If you count these both (line and pair of dots), you would see 16. Hydrogen can possess a maximum of 2 electrons, whereas other elements can possess a maximum of 8. Hydrogen atoms can be attached to the outside of oxygen molecules. The structure of \[{{\text{H}}_2}S{O_4}\] is shown below:

(b) The structural difference between white phosphorus and red phosphorus is stated below: White phosphorus consists of \[{P_4}\] molecules. Thus, white phosphorus exists as a \[{P_4}\] molecule in both solid as well as vapour states. On the other hand, the crystal structure of red phosphorus possesses a complicated network of bonding (it is polymeric) as it occurs as a chain of tetrahedral \[{P_4}\] units.

Note:
The main purpose to draw a Lewis dot structure is to determine the lone pair of electrons in molecules which helps to identify the formation of chemical bonds. Lewis structures can be drawn for molecules containing the covalent bonds as well as for coordination compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




