
A compound X (having vinegar like smell) when treated with ethanol in the presence of the acid Z, gives a compound Y which has a fruity smell, the reaction is:
${{C}_{2}}{{H}_{5}}OH+X\xrightarrow{Z}Y+{{H}_{2}}O$
(i) Identify Y and Z
(ii) Write the structural formula of X
(iii) Name the reaction
Answer
587.4k+ views
Hint: First find out what is compound X. It says that it has a vinegar smell. So, the other name of vinegar gives you an idea what is X. Then, see what type of reaction it undergoes when the given reactant is treated with X to give water and a compound with fruity odour.
Complete step by step solution:
As per the given question,
Compound X is having a vinegar smell.
We know vinegar is also called acetic acid.
So, the compound X having vinegar smell is acetic acid, whose chemical formula is $C{{H}_{3}}COOH$.
We can see that, in the given question the reactant given i.e. ${{C}_{2}}{{H}_{5}}OH$is an alcohol, having two carbon atoms and \[-OH\]as the functional group. This alcohol is called ethyl alcohol.
Now, let’s see which of the reaction processes produces water when an alcohol is treated with an acid.
So, we know that when an alcohol $(ROH)$[where R refers to a side chain or functional group] is treated with an acid $({{R}^{'}}COOH)$ it undergoes esterification forming an ester $(RCOO{{R}^{'}})$ and water. Esterification occurs in the presence of sulphuric acid (${{H}_{2}}S{{O}_{4}}$) that acts as a catalyst.
Thus, now we got the compound X and Z that are acetic acid and sulphuric acid respectively.
And here, let’s consider R as the ${{C}_{2}}{{H}_{5}}$ of ethyl alcohol and ${{R}^{'}}$as $C{{H}_{3}}$of acetic acid. So, after esterification, the product formed as explained above will be ${{C}_{2}}{{H}_{5}}-COO-C{{H}_{3}}$.
Looking forward to the chemical reaction that undergoes during esterification of the reactants we have, the following reaction is seen,
\[{{C}_{2}}{{H}_{5}}OH+C{{H}_{3}}COOH\xrightarrow{{{H}_{2}}S{{O}_{4}}}{{C}_{2}}{{H}_{5}}-COO-C{{H}_{3}}+{{H}_{2}}O\]
Thus, the product formed is ethyl acetate with chemical formula \[{{C}_{2}}{{H}_{5}}COOC{{H}_{3}}\] which will have a fruity smell.
So,
(i) The compound Y is ethyl acetate and Z is sulphuric acid.
(ii) Structural formula of X is,
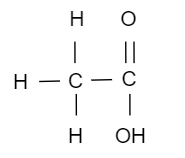
(iii) The reaction is called esterification.
Note: When a carboxylic acid is treated with an alcohol, esterification occurs in the presence of sulphuric acid (acts as a catalyst) which further forms ester and water. The ester formed generally has a pleasant characteristic i.e. fruity smell.
Complete step by step solution:
As per the given question,
Compound X is having a vinegar smell.
We know vinegar is also called acetic acid.
So, the compound X having vinegar smell is acetic acid, whose chemical formula is $C{{H}_{3}}COOH$.
We can see that, in the given question the reactant given i.e. ${{C}_{2}}{{H}_{5}}OH$is an alcohol, having two carbon atoms and \[-OH\]as the functional group. This alcohol is called ethyl alcohol.
Now, let’s see which of the reaction processes produces water when an alcohol is treated with an acid.
So, we know that when an alcohol $(ROH)$[where R refers to a side chain or functional group] is treated with an acid $({{R}^{'}}COOH)$ it undergoes esterification forming an ester $(RCOO{{R}^{'}})$ and water. Esterification occurs in the presence of sulphuric acid (${{H}_{2}}S{{O}_{4}}$) that acts as a catalyst.
Thus, now we got the compound X and Z that are acetic acid and sulphuric acid respectively.
And here, let’s consider R as the ${{C}_{2}}{{H}_{5}}$ of ethyl alcohol and ${{R}^{'}}$as $C{{H}_{3}}$of acetic acid. So, after esterification, the product formed as explained above will be ${{C}_{2}}{{H}_{5}}-COO-C{{H}_{3}}$.
Looking forward to the chemical reaction that undergoes during esterification of the reactants we have, the following reaction is seen,
\[{{C}_{2}}{{H}_{5}}OH+C{{H}_{3}}COOH\xrightarrow{{{H}_{2}}S{{O}_{4}}}{{C}_{2}}{{H}_{5}}-COO-C{{H}_{3}}+{{H}_{2}}O\]
Thus, the product formed is ethyl acetate with chemical formula \[{{C}_{2}}{{H}_{5}}COOC{{H}_{3}}\] which will have a fruity smell.
So,
(i) The compound Y is ethyl acetate and Z is sulphuric acid.
(ii) Structural formula of X is,

(iii) The reaction is called esterification.
Note: When a carboxylic acid is treated with an alcohol, esterification occurs in the presence of sulphuric acid (acts as a catalyst) which further forms ester and water. The ester formed generally has a pleasant characteristic i.e. fruity smell.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




