
A compound of Molecular formula ${{C}_{8}}{{H}_{8}}{{O}_{2}}$ reacts with acetophenone to form a single cross-aldol product in the presence of base. The same compound on reaction with conc. NaOH forms benzyl alcohol as one of the products. The structure of the compound is:
A.

B.

C.
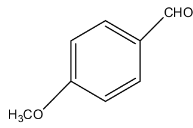
D.
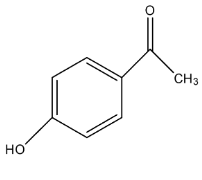




Answer
542.1k+ views
Hint: When a compound reacts with any compound in which cross-condensation occurs when the compound doesn’t have an alpha-carbon atom. When the compound reacts with conc. NaOH to form two products of which one is benzyl alcohol, then the process is called Cannizzaro reaction.
Complete step-by-step answer:
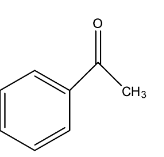
When a compound reacts with any compound in which cross-condensation occurs when the compound doesn’t have an alpha-carbon atom. So, the given compound has a formula ${{C}_{8}}{{H}_{8}}{{O}_{2}}$ reacts with acetophenone. Acetophenone has a structure:
The same compound with conc. NaOH will form one product benzyl alcohol, the structure of benzyl alcohol. So, we can say that the compound undergoes Cannizzaro's reaction, in which one compound is reduced to alcohol and one compound is reduced to carboxylate ion. For this, there must be an aldehyde group present on the compound.
From the given options, option (c) fits into the above two conditions.
The compound in option (c) is 4-Methoxybenaldehyde, and it also even doesn't have an alpha-carbon atom. The reaction of cross-aldol condensation is:
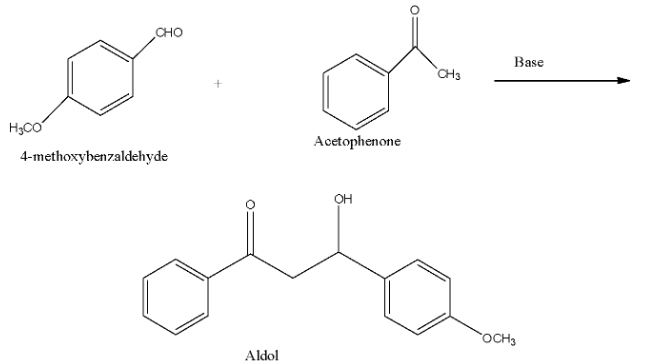
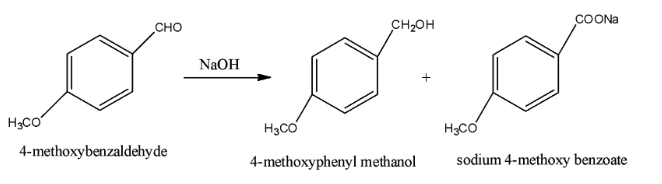
Now, the Cannizzaro reaction of 4-methoxy benzaldehyde is shown below. The two products are 4-methoxyphenyl methanol and sodium 4-methoxy benzoate.
Therefore, the correct answer is an option (c).
Note: If the compound has an alpha-carbon atom, and when it undergoes cross-aldol condensation then there will be the formation of two products. When the aldol-condensation takes place between one aliphatic aldehyde and one aromatic aldehyde then the reaction is called the Claisen reaction.
Complete step-by-step answer:
When a compound reacts with any compound in which cross-condensation occurs when the compound doesn’t have an alpha-carbon atom. So, the given compound has a formula ${{C}_{8}}{{H}_{8}}{{O}_{2}}$ reacts with acetophenone. Acetophenone has a structure:

The same compound with conc. NaOH will form one product benzyl alcohol, the structure of benzyl alcohol. So, we can say that the compound undergoes Cannizzaro's reaction, in which one compound is reduced to alcohol and one compound is reduced to carboxylate ion. For this, there must be an aldehyde group present on the compound.
From the given options, option (c) fits into the above two conditions.
The compound in option (c) is 4-Methoxybenaldehyde, and it also even doesn't have an alpha-carbon atom. The reaction of cross-aldol condensation is:

Now, the Cannizzaro reaction of 4-methoxy benzaldehyde is shown below. The two products are 4-methoxyphenyl methanol and sodium 4-methoxy benzoate.

Therefore, the correct answer is an option (c).
Note: If the compound has an alpha-carbon atom, and when it undergoes cross-aldol condensation then there will be the formation of two products. When the aldol-condensation takes place between one aliphatic aldehyde and one aromatic aldehyde then the reaction is called the Claisen reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




