
A carbonyl compound P, which gives positive iodoform test, undergoes reaction with $C{{H}_{3}}MgBr$, followed by dehydration to give an olefin Q. The ozonolysis of Q gives rise to a dicarbonyl compound R, which undergoes intramolecular aldol condensation reaction to predominantly give S.
The structure of the carbonyl compound P is:
\[P\xrightarrow[\begin{smallmatrix}
2.H{}^{+},\text{ }{{H}_{2}}O \\
3.{{H}_{2}}S{{O}_{4}},\Delta
\end{smallmatrix}]{1.MeMgBr}Q\xrightarrow[2.Zn,{{H}_{2}}O]{1.{{O}_{3}}}R\xrightarrow[2.\text{ }\Delta ]{1.O{{H}^{-}}}S\]
A.
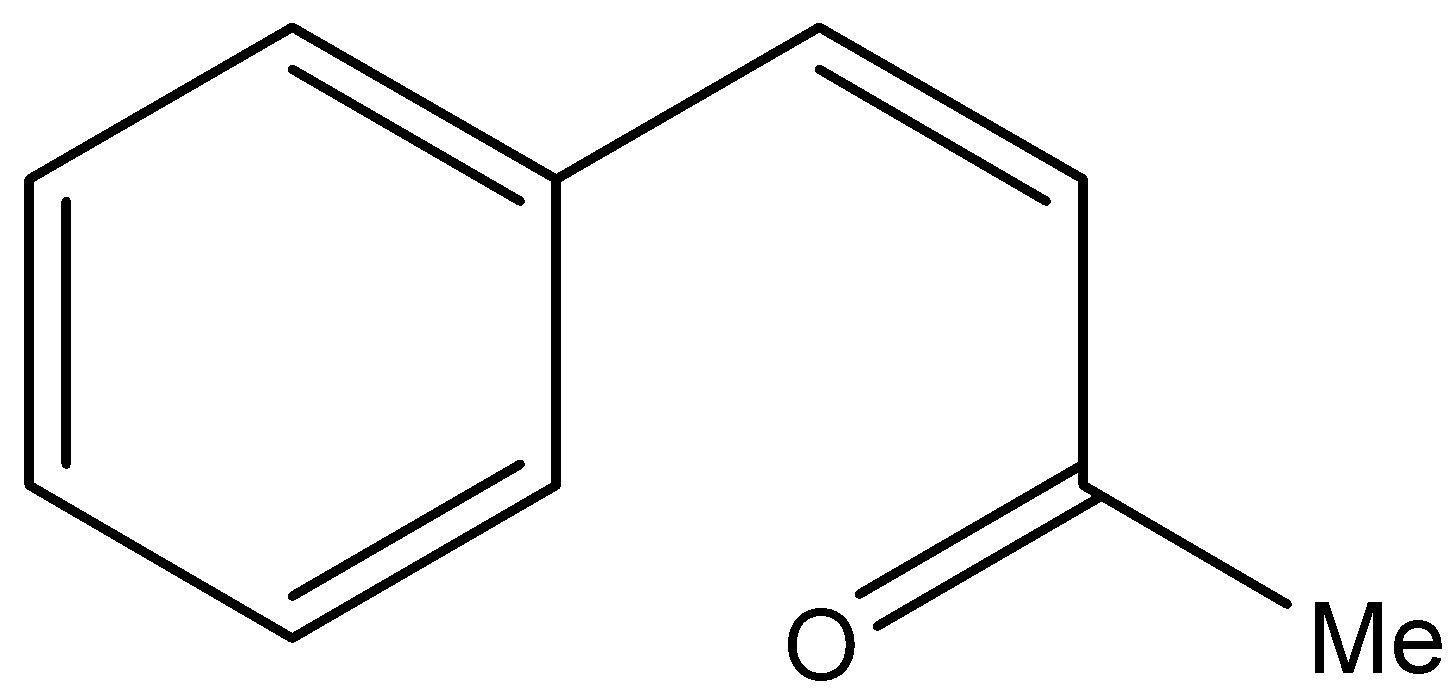
B.
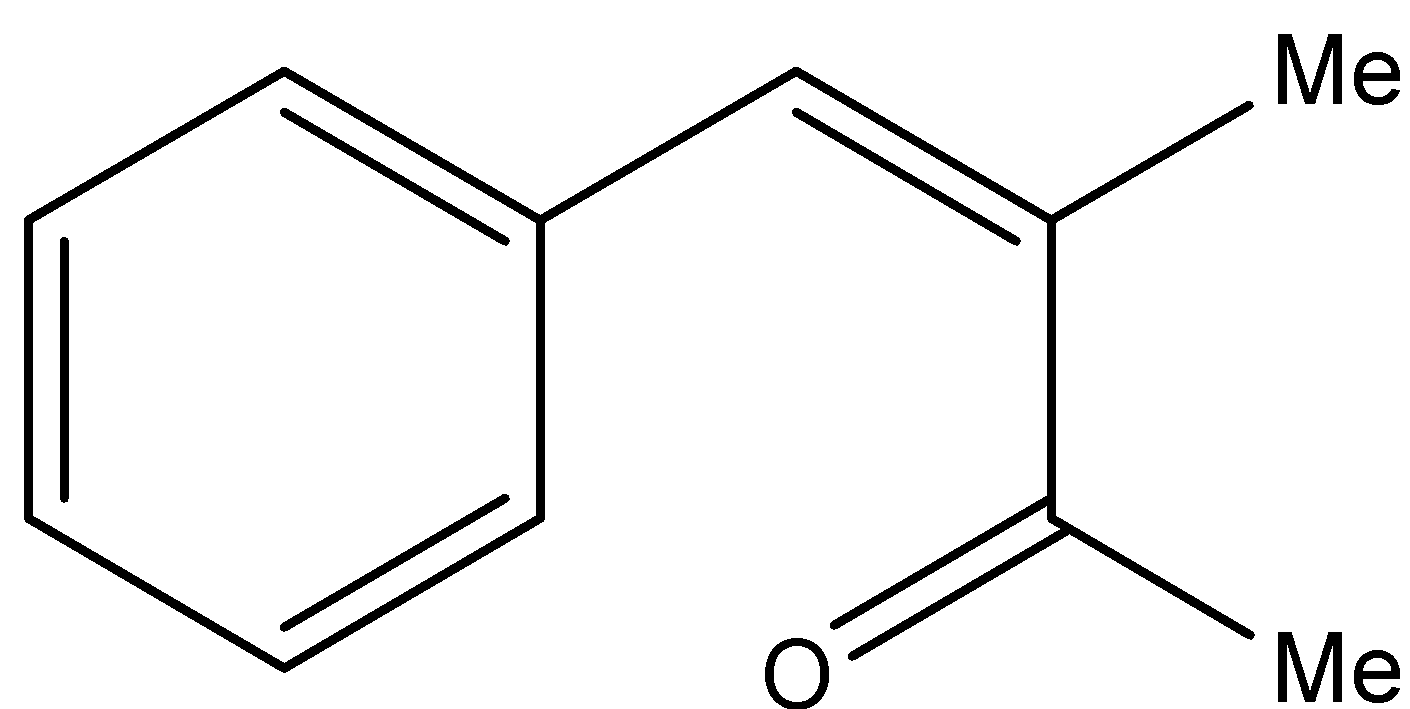
C.
D.




Answer
577.8k+ views
Hint: Methyl ketones respond to iodoform test and form respective carboxylic acid and iodoform as the products. It is a test used to find the presence of methyl ketones in the given compounds. Generally methyl ketones react with Grignard reagent and form respective alcohols as the products.
Complete answer:
- In the question it is mentioned that the compound-P gives Iodoform test, means compound-P must be a methyl ketone. Then from the given options P will be either A or B, because Option A and B only contains methyl ketones in their structure.
- Now by taking structures from option A and option B we will proceed and write the products formed as per the question. Later will decide which option will be correct.
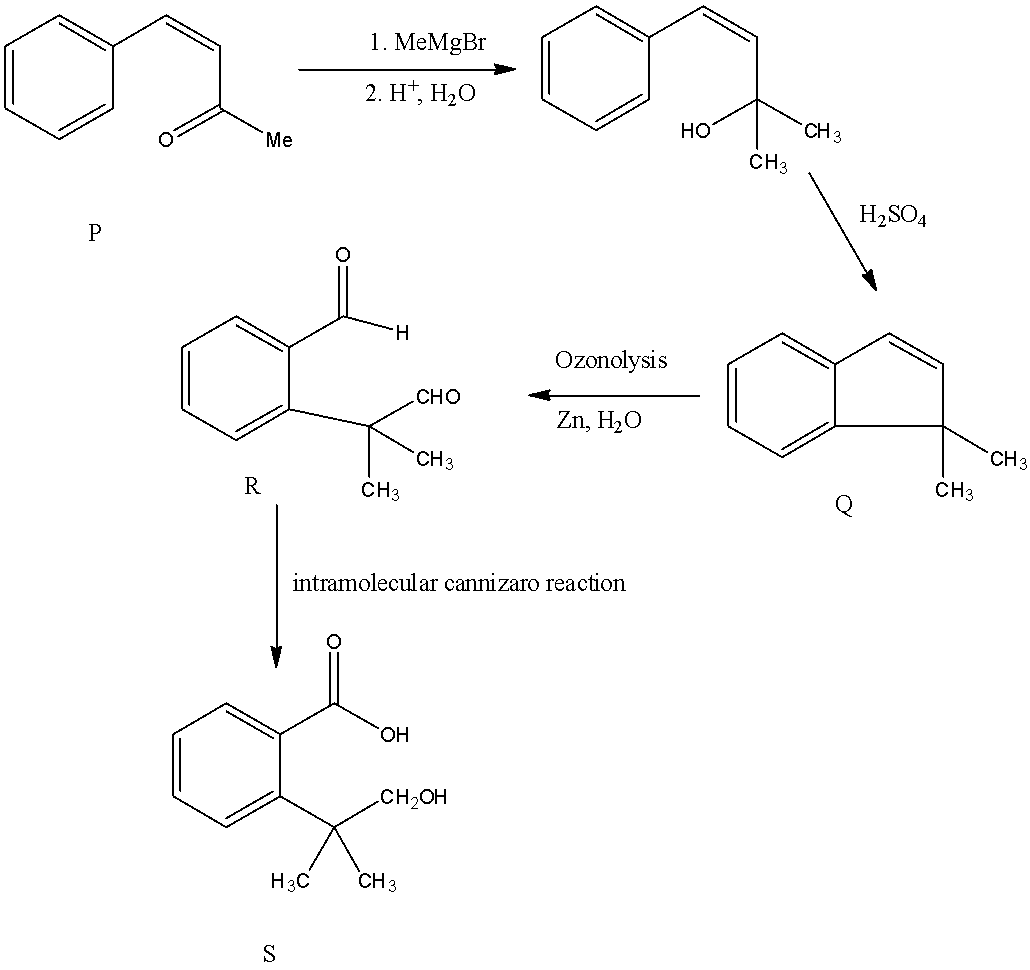
- If we are going to take Option A as P the following reaction takes place.
- If we are going to take Option B as P the following reaction takes place.
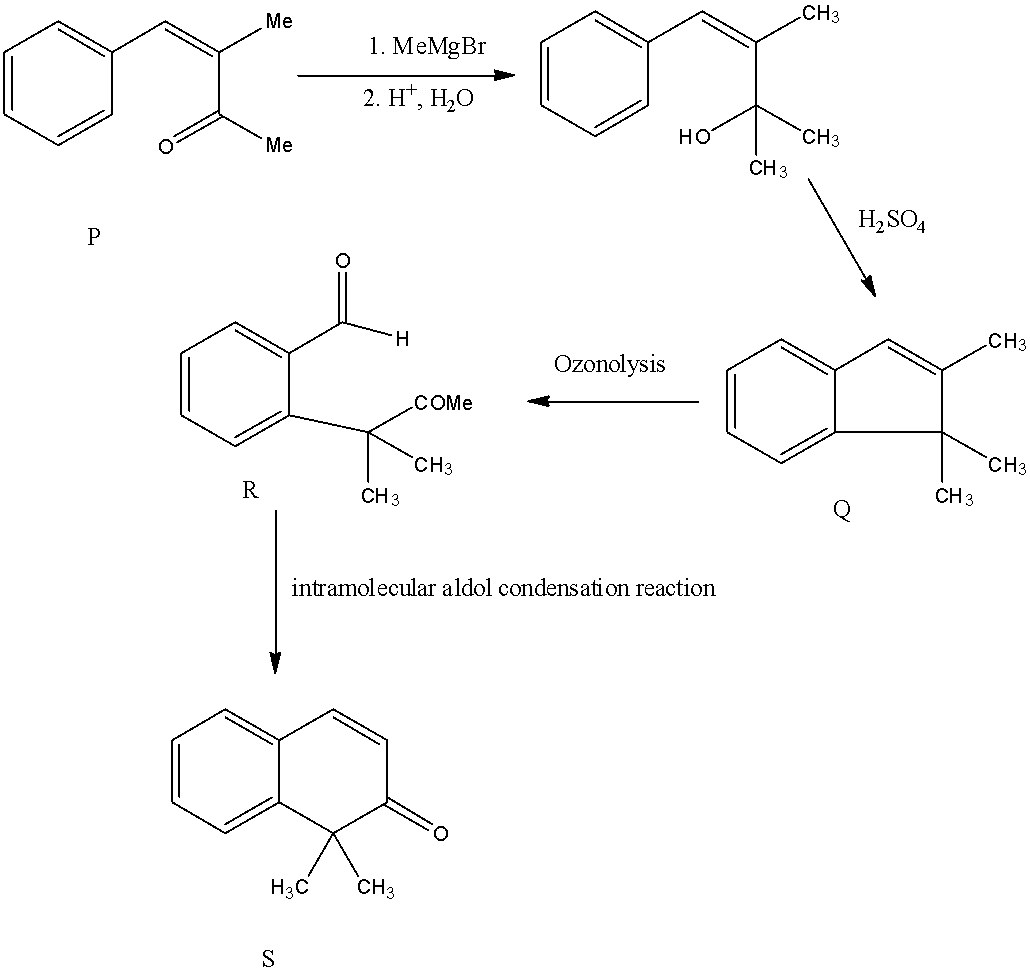
- But compound ‘R’ undergoes intramolecular aldol condensation and forms a cyclic compound (S), it only happens if we consider compound P is Option B.
- If we take compound P is option B, then in intermolecular aldol condensation it won’t form a cyclic compound (S)
Therefore the compound P is option B.
Note:
If we are going to take option A is compound P then the compound R formed as an intermediate product undergo intramolecular Cannizaro reaction and forms a carboxylic group and an alcohol in compound S. but in the question it is clearly mentioned the compound R undergoes intramolecular aldol condensation reaction means the Compound P is option B only.
Complete answer:
- In the question it is mentioned that the compound-P gives Iodoform test, means compound-P must be a methyl ketone. Then from the given options P will be either A or B, because Option A and B only contains methyl ketones in their structure.
- Now by taking structures from option A and option B we will proceed and write the products formed as per the question. Later will decide which option will be correct.
- If we are going to take Option A as P the following reaction takes place.

- If we are going to take Option B as P the following reaction takes place.

- But compound ‘R’ undergoes intramolecular aldol condensation and forms a cyclic compound (S), it only happens if we consider compound P is Option B.
- If we take compound P is option B, then in intermolecular aldol condensation it won’t form a cyclic compound (S)
Therefore the compound P is option B.
Note:
If we are going to take option A is compound P then the compound R formed as an intermediate product undergo intramolecular Cannizaro reaction and forms a carboxylic group and an alcohol in compound S. but in the question it is clearly mentioned the compound R undergoes intramolecular aldol condensation reaction means the Compound P is option B only.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




