
When a carbohydrate reacts with $NaB{H_4}$, the product is an:
A) Alditol
B) Aldaric acid
C) Aldonic acid
D) Aglycone
Answer
585.3k+ views
Hint: The reagent given in the question sodium borohydride i.e. $NaB{H_4}$ is a reducing agent. One can think about which functional group in the structures can undergo the reduction by reducing agents. One can analyze the product after reduction and relate the options given.
Complete step by step answer:
1) First of all we will learn about the reagent $NaB{H_4}$ i.e. Sodium borohydride which is a good reducing agent and it is very effective for the reduction of aldehydes and ketones to convert into alcohols.
2) There is a carbonyl group present in the carbohydrates which will get reduced to the alcohol group by the action of $NaB{H_4}$.
3) Now let us see the reaction scheme as follows,
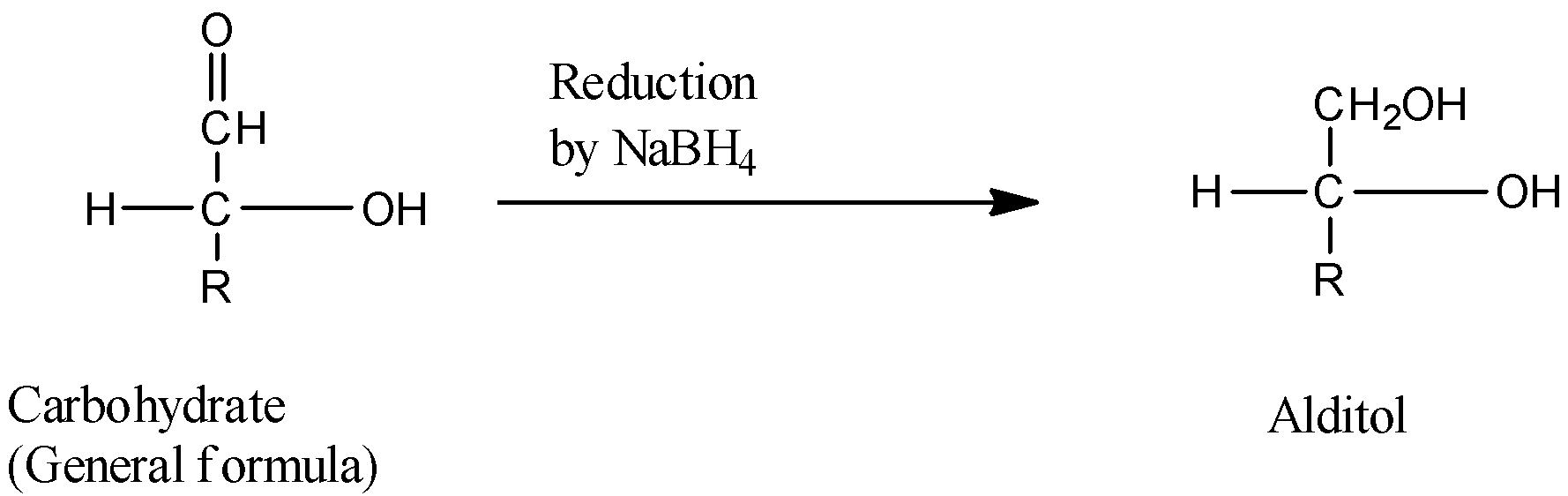
4) Therefore, when a carbohydrate reacts with $NaB{H_4}$ it will form alditol .
So, the correct answer is Option A .
Additional Information:
The structure of carbohydrates can contain hydroxyl alcohol groups i.e. alcohol group, aldehydes and ketones, ethers. Basically, the carbohydrates are structures that have chains or polymers of basic sugar molecules such as glucose, galactose, and fructose. To identify which functional groups are present in the carbohydrate structure one must look at the functional groups which are present in the more basic building blocks of that structure. Saccharides which are a type of carbohydrates are composed of only three atoms of carbon, hydrogen, and oxygen.
Note:
The reducing reagent $NaB{H_4}$ is used instead of $LiAl{H_4}$ because it is usually not suitable due to its incompatibility with the polar solvents. When sodium borohydride reacts with aldehyde it gives primary alcohol and with a ketone it will give secondary alcohol. The compound alditol is also called as a carbohydrate that is an acyclic polyol.
Complete step by step answer:
1) First of all we will learn about the reagent $NaB{H_4}$ i.e. Sodium borohydride which is a good reducing agent and it is very effective for the reduction of aldehydes and ketones to convert into alcohols.
2) There is a carbonyl group present in the carbohydrates which will get reduced to the alcohol group by the action of $NaB{H_4}$.
3) Now let us see the reaction scheme as follows,

4) Therefore, when a carbohydrate reacts with $NaB{H_4}$ it will form alditol .
So, the correct answer is Option A .
Additional Information:
The structure of carbohydrates can contain hydroxyl alcohol groups i.e. alcohol group, aldehydes and ketones, ethers. Basically, the carbohydrates are structures that have chains or polymers of basic sugar molecules such as glucose, galactose, and fructose. To identify which functional groups are present in the carbohydrate structure one must look at the functional groups which are present in the more basic building blocks of that structure. Saccharides which are a type of carbohydrates are composed of only three atoms of carbon, hydrogen, and oxygen.
Note:
The reducing reagent $NaB{H_4}$ is used instead of $LiAl{H_4}$ because it is usually not suitable due to its incompatibility with the polar solvents. When sodium borohydride reacts with aldehyde it gives primary alcohol and with a ketone it will give secondary alcohol. The compound alditol is also called as a carbohydrate that is an acyclic polyol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




