
A capillary tube made of glass of radius 0.15 mm is dipped vertically in a beaker filled with methylene iodide (surface tension = $0.05{\rm{ N}}{\rm{.}}{{\rm{m}}^{ - 1}}$, density = \[667{\rm{ kg}}{\rm{.}}{{\rm{m}}^{ - 3}}\]) which rises to height h in the tube. It is observed that the two tangents drawn from liquid-glass interfaces (from opp. sides of the capillary) make an angle of with one another. Then h is close to (g = $10{\rm{ m}}{{\rm{s}}^{ - 2}}$)
A. 0.172 m
B. 0.049 m
C. 0.087 m
D. 0.137 m
Answer
334.2k+ views
Hint:When a liquid is filled inside a capillary tube, that liquid rises into the capillary due to the surface tension of the liquid. This rise in the liquid into the capillary depends upon the density of the liquid, surface tension between the liquid and the capillary tube, radius of the capillary tube, and contact angle.
Formula used:
The formula to find the height in the capillary tube is,
\[h = \dfrac{{2T\cos \theta }}{{r\rho g}}\]
Where,
\[T\] is surface tension of methylene iodide
\[\theta \] is the angle of contact
\[r\] is radius of the capillary tube
\[\rho \] is density of methylene iodide
\[g\] is the acceleration due to gravity
Complete step by step solution:
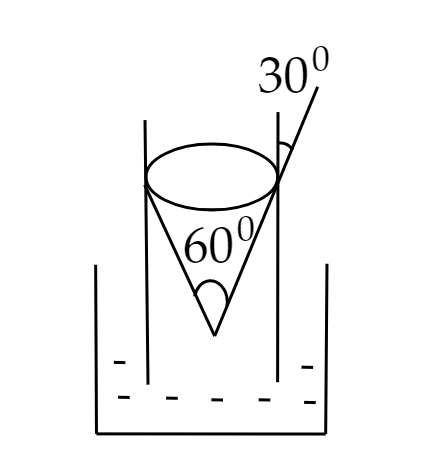
Image: A capillary tube dipped in a beaker filled with methylene iodide
Capillary tubes are very thin tubes made of a rigid material, such as plastic or glass in which a liquid flow up into the tubes against gravity in a process called capillary action (capillarity) and it will occur due to the cohesive force between the molecules of liquid and adhesive force between the surface of the tube and the liquid. The contact angle of liquid is defined as the angle it makes with the walls of the container and it depends both on the liquid as well as the container.
To find the height in the capillary tube we have,
\[h = \dfrac{{2T\cos \theta }}{{r\rho g}}\]
\[\Rightarrow h = \dfrac{{2 \times 0.05 \times \cos {{30}^0}}}{{0.15 \times {{10}^{ - 3}} \times 667 \times 10}}\]
\[\Rightarrow h = \dfrac{{2 \times 0.05 \times \left( {\dfrac{{\sqrt 3 }}{2}} \right)}}{{0.15 \times {{10}^{ - 2}} \times 667}}\]
\[\Rightarrow h = 0.0866\,m\]
\[ \therefore h \simeq 0.087m\]
Therefore, the closest value of h is 0.087 m.
Hence, Option A is the correct answer
Note: Due to the water molecules' attachment to the capillary tube's glass walls, water rises inside of it. The result of this adhesion and surface tension in the water is a phenomenon known as capillarity, which has a distinctive concave surface.
Formula used:
The formula to find the height in the capillary tube is,
\[h = \dfrac{{2T\cos \theta }}{{r\rho g}}\]
Where,
\[T\] is surface tension of methylene iodide
\[\theta \] is the angle of contact
\[r\] is radius of the capillary tube
\[\rho \] is density of methylene iodide
\[g\] is the acceleration due to gravity
Complete step by step solution:

Image: A capillary tube dipped in a beaker filled with methylene iodide
Capillary tubes are very thin tubes made of a rigid material, such as plastic or glass in which a liquid flow up into the tubes against gravity in a process called capillary action (capillarity) and it will occur due to the cohesive force between the molecules of liquid and adhesive force between the surface of the tube and the liquid. The contact angle of liquid is defined as the angle it makes with the walls of the container and it depends both on the liquid as well as the container.
To find the height in the capillary tube we have,
\[h = \dfrac{{2T\cos \theta }}{{r\rho g}}\]
\[\Rightarrow h = \dfrac{{2 \times 0.05 \times \cos {{30}^0}}}{{0.15 \times {{10}^{ - 3}} \times 667 \times 10}}\]
\[\Rightarrow h = \dfrac{{2 \times 0.05 \times \left( {\dfrac{{\sqrt 3 }}{2}} \right)}}{{0.15 \times {{10}^{ - 2}} \times 667}}\]
\[\Rightarrow h = 0.0866\,m\]
\[ \therefore h \simeq 0.087m\]
Therefore, the closest value of h is 0.087 m.
Hence, Option A is the correct answer
Note: Due to the water molecules' attachment to the capillary tube's glass walls, water rises inside of it. The result of this adhesion and surface tension in the water is a phenomenon known as capillarity, which has a distinctive concave surface.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




