
A. Bakelite is a thermosetting polymer.
R. It can be melted again and again without any change.
(A) Both A and R are correct and R is the correct explanation of the A.
(B) Both A and R are correct but R is not the correct explanation of the A.
(C) A is correct but R is incorrect.
(D) A is incorrect but R is correct.
Answer
582.9k+ views
Hint: A thermosetting polymer, upon heating is hardened and cannot be softened again.
A polymer, which undergoes extensive cross linking upon heating, cannot be melted again and again without any change.
Complete answer:
Bakelite is also called phenol formaldehyde resin. It is obtained by elimination reaction between phenol and formaldehyde.
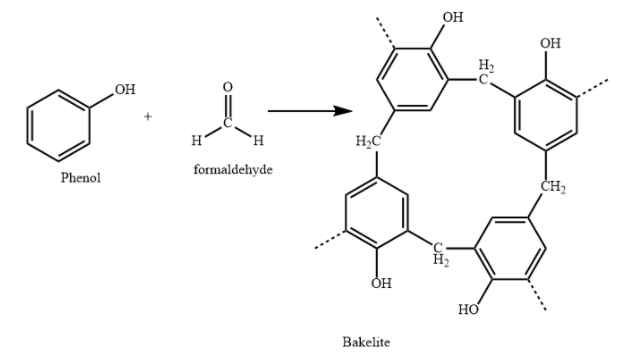
Bakelite contains cross links, or heavily branched polymer chains. Bakelite polymer, upon heating is hardened and cannot be softened again.
Hence, it is a thermosetting polymer. Hence, the assertion is correct.
When bakelite is heated, the degree of cross linking significantly increases. So the polymer mass becomes infusible. It is not possible to get back the original shape after melting. Hence, it cannot be reused.
Hence, the bakelite polymer cannot be melted again and again without any change.
Hence, the reason is incorrect.
Hence, the option (C) is the correct answer.
Additional Information: Bakelite has high resistance to that and electricity. Hence, it is used in automotive components. It also has some industrial applications. Bakelite has very good insulating properties. Hence, it is used in making electrical switches. Bakelite is also used in frying pans and other kitchenware products. Also some toys and jewelry articles are made from bakelite.
Note: A thermoplastic polymer can be repeatedly softened by heating and hardened by cooling. Hence, thermoplastic polymers can be used again and again. Polythene, polypropylene etc are thermoplastic polymers.
A polymer, which undergoes extensive cross linking upon heating, cannot be melted again and again without any change.
Complete answer:
Bakelite is also called phenol formaldehyde resin. It is obtained by elimination reaction between phenol and formaldehyde.

Bakelite contains cross links, or heavily branched polymer chains. Bakelite polymer, upon heating is hardened and cannot be softened again.
Hence, it is a thermosetting polymer. Hence, the assertion is correct.
When bakelite is heated, the degree of cross linking significantly increases. So the polymer mass becomes infusible. It is not possible to get back the original shape after melting. Hence, it cannot be reused.
Hence, the bakelite polymer cannot be melted again and again without any change.
Hence, the reason is incorrect.
Hence, the option (C) is the correct answer.
Additional Information: Bakelite has high resistance to that and electricity. Hence, it is used in automotive components. It also has some industrial applications. Bakelite has very good insulating properties. Hence, it is used in making electrical switches. Bakelite is also used in frying pans and other kitchenware products. Also some toys and jewelry articles are made from bakelite.
Note: A thermoplastic polymer can be repeatedly softened by heating and hardened by cooling. Hence, thermoplastic polymers can be used again and again. Polythene, polypropylene etc are thermoplastic polymers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




