
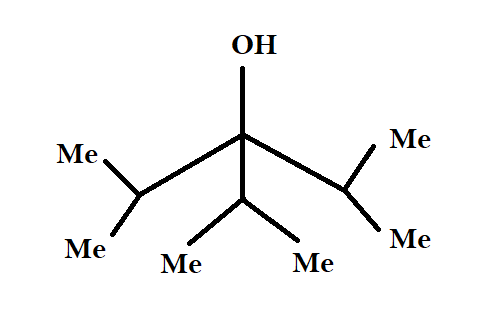
A $3^\circ $ alcohol as shown can be obtained by the reaction of ketone (diisopropyl ketone) and__________.
A. isopropyl magnesium bromide
B. isopropyl lithium
C. diisopropyl cadmium
D. diisopropyl zinc

Answer
562.8k+ views
Hint:If we draw the structures of our initial compound and final compound, we will get an idea of what needs to happen, or which groups need to get added. Also, the ketone needs to be reduced into an alcohol, suggesting that we need a reducing agent.
Complete step by step answer:
First let us examine the structure of our reactant, diisopropyl ketone. Note that $Me$ stands for methyl group, that is, $C{H_3} - $ group. Our initial compound is a ketone with two isopropyl (${(C{H_3})_2} - CH - $)groups on either side of a keto ($C = O$) group. Comparing the structure of our reactant and product side by side, we can see what actually needs to happen.


An isopropyl group needs to come as a nucleophile and has to be added below the central carbon atom, and also, the keto group should get reduced to an alcohol. So, our reagent should be a reducing agent which has only one isopropyl group.
Also, if we notice the structure of diisopropyl ketone, we see that it has two bulky isopropyl groups on either side of the central carbon atom. This makes the compound very unreactive towards nucleophilic substitution, as the incoming isopropyl group and the already present bulky isopropyl groups tend to repel each other. This means that we need a reagent which has low steric hindrance, and can produce a strong nucleophilic isopropyl group.
Options C and D are very bulky compounds and also have two isopropyl groups, which is not needed in this case. Therefore, we can directly cancel them off.
Now between isopropyl magnesium bromide and isopropyl lithium, we know that lithium being one of the most electropositive (tending to repel electrons) metals, can easily generate a strong nucleophile (species rich in electrons). Also, it is less bulky than isopropyl magnesium bromide and can thus, react easily.
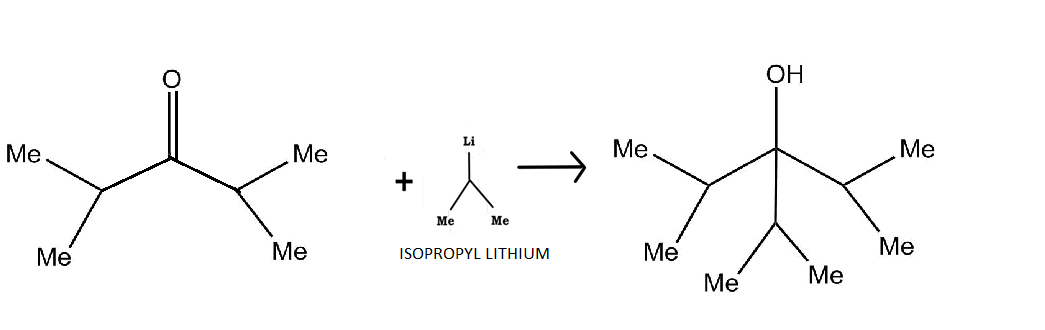
Hence, the correct option is B.
Note:
This reaction is nucleophilic addition because of the fact that the carbonyl carbon (carbon atom containing keto group) is electropositive, due to the high electronegativity of oxygen. Thus, if we examine the reaction mechanism, in the first step the double bond breaks and one pair of electrons goes to oxygen. This makes the nucleophile (isopropyl group of isopropyl lithium) attack the carbonyl carbon and it gets attached there. The oxygen then gets protonated (addition of ${H^ + }$ ion) to form the $3^\circ $ alcohol.
Complete step by step answer:
First let us examine the structure of our reactant, diisopropyl ketone. Note that $Me$ stands for methyl group, that is, $C{H_3} - $ group. Our initial compound is a ketone with two isopropyl (${(C{H_3})_2} - CH - $)groups on either side of a keto ($C = O$) group. Comparing the structure of our reactant and product side by side, we can see what actually needs to happen.


An isopropyl group needs to come as a nucleophile and has to be added below the central carbon atom, and also, the keto group should get reduced to an alcohol. So, our reagent should be a reducing agent which has only one isopropyl group.
Also, if we notice the structure of diisopropyl ketone, we see that it has two bulky isopropyl groups on either side of the central carbon atom. This makes the compound very unreactive towards nucleophilic substitution, as the incoming isopropyl group and the already present bulky isopropyl groups tend to repel each other. This means that we need a reagent which has low steric hindrance, and can produce a strong nucleophilic isopropyl group.
Options C and D are very bulky compounds and also have two isopropyl groups, which is not needed in this case. Therefore, we can directly cancel them off.
Now between isopropyl magnesium bromide and isopropyl lithium, we know that lithium being one of the most electropositive (tending to repel electrons) metals, can easily generate a strong nucleophile (species rich in electrons). Also, it is less bulky than isopropyl magnesium bromide and can thus, react easily.

Hence, the correct option is B.
Note:
This reaction is nucleophilic addition because of the fact that the carbonyl carbon (carbon atom containing keto group) is electropositive, due to the high electronegativity of oxygen. Thus, if we examine the reaction mechanism, in the first step the double bond breaks and one pair of electrons goes to oxygen. This makes the nucleophile (isopropyl group of isopropyl lithium) attack the carbonyl carbon and it gets attached there. The oxygen then gets protonated (addition of ${H^ + }$ ion) to form the $3^\circ $ alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




