
When 2-ethylanthraquinone dissolved in a mixture of benzene and cyclohexanol is oxidized, the product is:
A. Ethanol
B. Hydrogen peroxide
C. Anthracene
D. None of these
Answer
583.2k+ views
Hint: 2-ethylanthraquinone is an organic compound that is a derivative of anthraquinone. This pale yellow solid is used in the industrial production of hydrogen peroxide (${H_2}{O_2}$ ).
Complete step by step answer:
Whenever an alcohol is oxidized in presence of a suitable oxidising agent, it yields either an aldehyde or acetone as the product. 2-ethyl anthraquinone is a structure in which there are two alcoholic groups present at the center of the anthracene ring along with one ethyl group present at the second carbon atom of the adjacent ring in the anthracene molecule itself. When this compound is oxidized in the presence of air, it produces a very light yellow colored compound known as the 2-ethylanthraquinone.
When 2-ethylanthraquinone dissolved in a mixture of benzene and cyclohexanol is oxidized, the product is as follows:
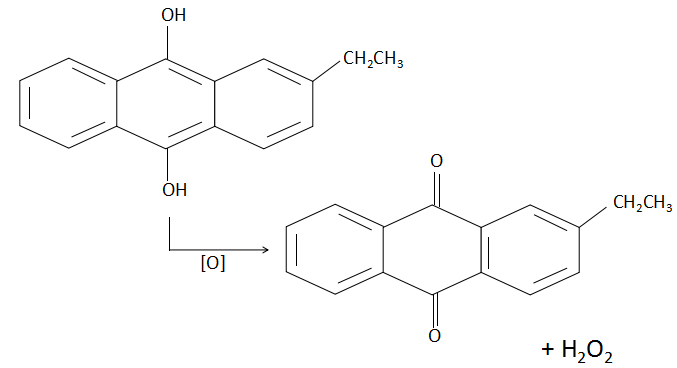
As we can clearly see that the extra product that we gain besides the 2-ethylanthraquinone molecule is hydrogen peroxide. This method is also a quantitative method employed in the industries for the production of ${H_2}{O_2}$ . The reverse of this process is known as hydrogenation which is catalysed by a palladium catalyst.
So, the correct answer is Option B .
Note:
Hydrogen peroxide is a chemical compound with the formula ${H_2}{O_2}$ . In its pure form, it is a very pale blue colored liquid. It is found to be slightly more viscous than water. Hydrogen peroxide is the simplest peroxide. It is used as an oxidizer, bleaching agent, and antiseptic. It has an open book structure with a single moiety of oxygen- oxygen bond in it.
Complete step by step answer:
Whenever an alcohol is oxidized in presence of a suitable oxidising agent, it yields either an aldehyde or acetone as the product. 2-ethyl anthraquinone is a structure in which there are two alcoholic groups present at the center of the anthracene ring along with one ethyl group present at the second carbon atom of the adjacent ring in the anthracene molecule itself. When this compound is oxidized in the presence of air, it produces a very light yellow colored compound known as the 2-ethylanthraquinone.
When 2-ethylanthraquinone dissolved in a mixture of benzene and cyclohexanol is oxidized, the product is as follows:

As we can clearly see that the extra product that we gain besides the 2-ethylanthraquinone molecule is hydrogen peroxide. This method is also a quantitative method employed in the industries for the production of ${H_2}{O_2}$ . The reverse of this process is known as hydrogenation which is catalysed by a palladium catalyst.
So, the correct answer is Option B .
Note:
Hydrogen peroxide is a chemical compound with the formula ${H_2}{O_2}$ . In its pure form, it is a very pale blue colored liquid. It is found to be slightly more viscous than water. Hydrogen peroxide is the simplest peroxide. It is used as an oxidizer, bleaching agent, and antiseptic. It has an open book structure with a single moiety of oxygen- oxygen bond in it.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




