
When $1-$butene is mixed with an excess of bromine, the expected reaction product is
A. $1,2-$dibromobutane
B. $1,1-$dibromoethane
C. 2,2-dibromobutane
D. Per Bromobutane
Answer
360.9k+ views
Hint: Addition reactions provide a path to add new substituents to a hydrocarbon chain and hence synthesise new derivatives of the mother alkene. When alkene reacts with bromine an addition reaction occurs very rapidly. Two bromine atoms are added across the double bond through the formation of a cyclic intermediate.
Complete Step by Step Answer:
$1-$butane is a type of asymmetrical alkene. The structure of this compound is:

$1-butene$ reacts with an excess of bromine, and an addition reaction occurs. When halogen (Here bromine) approaches a double bond of the alkene, there is a repulsion between electrons in the double bond of alkene and electrons in the bromine molecule resulting in a polarisation of the bromine-bromine ($Br-Br$) bond.
Thereby one halogen obtains a positive charge through heterolytic cleavage and acts as an electrophile in addition to a reaction with an alkene.
In the first step, the polarisation of the Br-Br atom occurs and through heterolytic cleavage, a cyclic intermediate is formed with two carbons from an alkene.
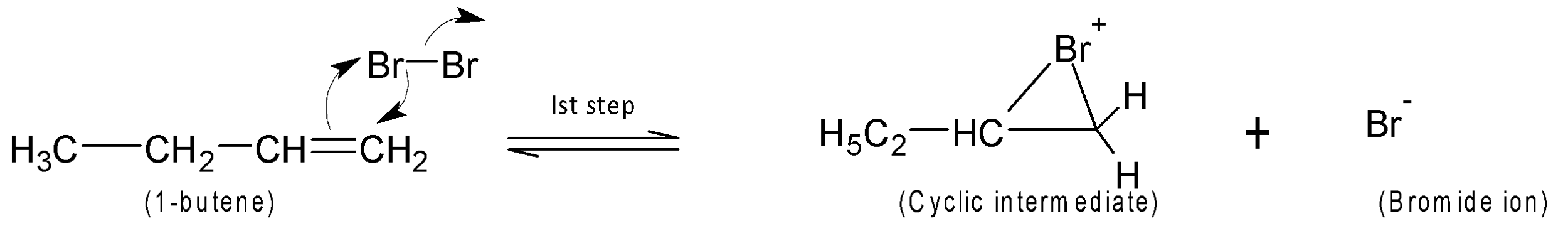
In the second step, the nucleophile bromide anion attacks from the back side at either carbon of the bridged bromonium ion. This ring-opening step with two halogens attached across the double bond of an alkene. The addition reaction is stereoselective and here trans addition of bromine gives the final product.
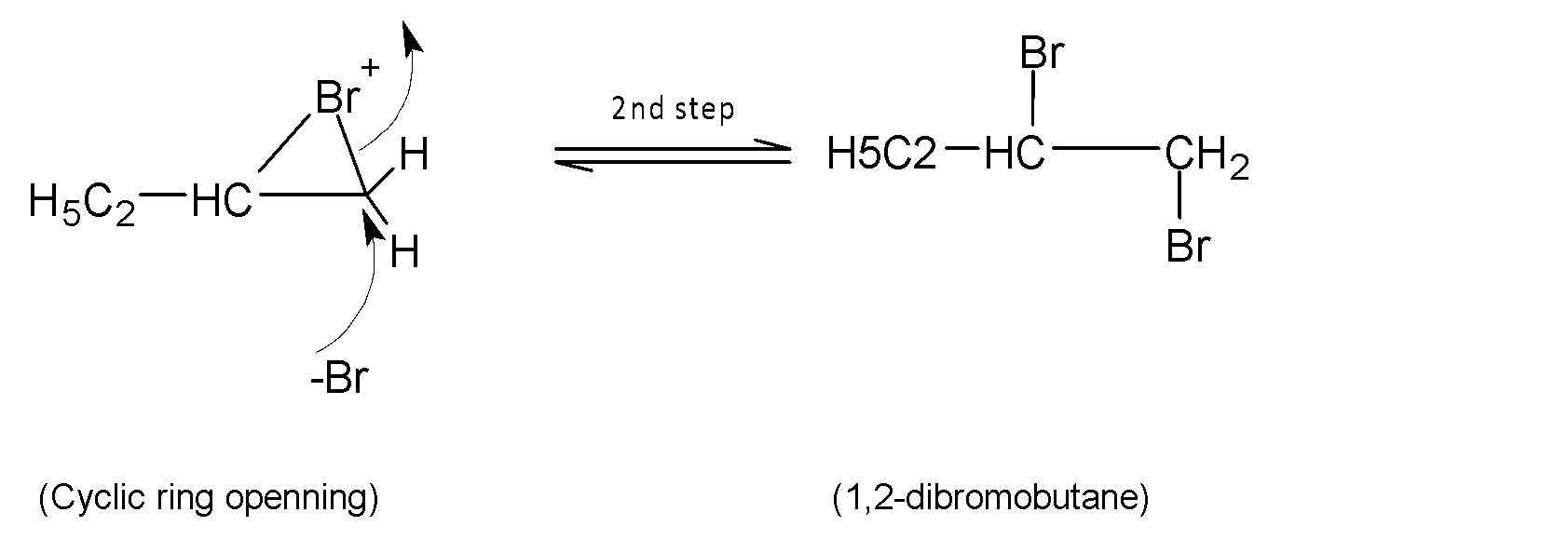
Therefore, the expected reaction product is $1,2-$dibromobutane.
Thus, option (A) is correct.
Note: The addition reaction between an alkene and halogen molecule gives optically inactive achiral products or a racemic mixture from the optically inactive starting material. This is possible because a nucleophilic halide anion can attack any carbon from the opposite side of the ring.
Complete Step by Step Answer:
$1-$butane is a type of asymmetrical alkene. The structure of this compound is:

$1-butene$ reacts with an excess of bromine, and an addition reaction occurs. When halogen (Here bromine) approaches a double bond of the alkene, there is a repulsion between electrons in the double bond of alkene and electrons in the bromine molecule resulting in a polarisation of the bromine-bromine ($Br-Br$) bond.
Thereby one halogen obtains a positive charge through heterolytic cleavage and acts as an electrophile in addition to a reaction with an alkene.
In the first step, the polarisation of the Br-Br atom occurs and through heterolytic cleavage, a cyclic intermediate is formed with two carbons from an alkene.
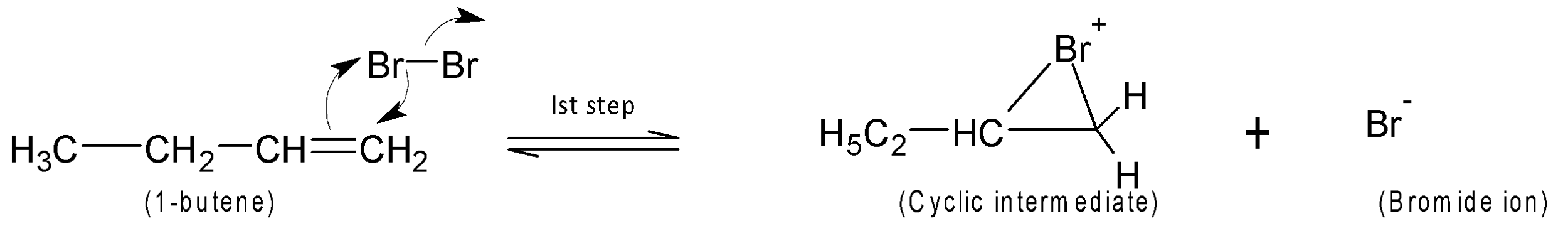
In the second step, the nucleophile bromide anion attacks from the back side at either carbon of the bridged bromonium ion. This ring-opening step with two halogens attached across the double bond of an alkene. The addition reaction is stereoselective and here trans addition of bromine gives the final product.

Therefore, the expected reaction product is $1,2-$dibromobutane.
Thus, option (A) is correct.
Note: The addition reaction between an alkene and halogen molecule gives optically inactive achiral products or a racemic mixture from the optically inactive starting material. This is possible because a nucleophilic halide anion can attack any carbon from the opposite side of the ring.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Difference Between Plant Cell and Animal Cell




