




Real-World Uses of Carnot Theorem in Thermodynamics
Carnot theorem, often known as Carnot's rule, was created in 1824 by Nicolas Léonard Sadi Carnot and is a theory that describes the greatest efficiency that any heat engine may achieve. A Carnot engine's efficiency is solely determined by the temperatures of its hot and cold reservoirs.
Carnot Theorem
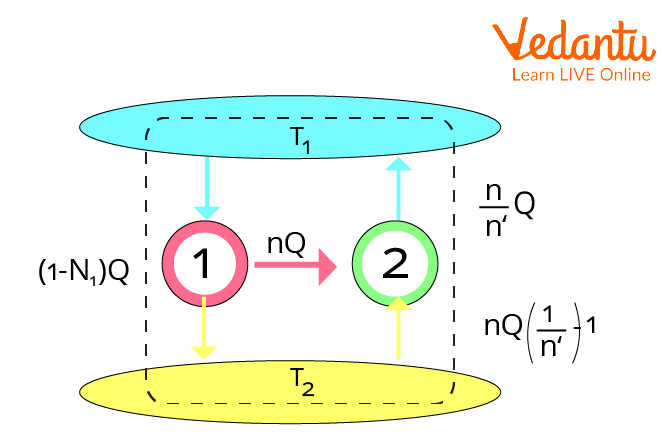
This theorem has the conclusion that every Carnot heat engine operating across two heat reservoirs is equally efficient, irrespective of the working substance or operation details.
History of Nicolas Léonard Sadi Carnot
Carnot's theorem was given in 1824 by Nicolas Léonard Sadi Carnot, a French Army mechanical engineer, military scientist, and physicist known as the "Father of Thermodynamics."

Nicolas Léonard Sadi Carnot (1 June 1796 – 24 August 1832)
The Carnot theorem is a consequence of the second law of thermodynamics. It was historically founded on contemporary caloric theory and predated the formation of the second law.
Carnot Theorem Statement
According to Carnot's theorem, the heat engine's efficiency has no boundaries; it can be as high as it can be dependent on the hot and cold reservoir temperatures.
\[{\eta _{\max }} = {\eta _{carnot}}=1-\dfrac {T_C}{T_H}\]\[\]
Where,
\[T_C\] - Absolute temperature of the cold source
\[T_H\] - Absolute temperature of the hot source
Carnot’s Theorem Proof
Consider a heat engine that draws heat Q1 from a heat reservoir at T1, delivers work W, and dumps heat Q2 into a heat sink at T2.
The heat engine operates in cycles( each of which includes two isothermal and two adiabatic processes), meaning it takes in heat Q1, performs work W, dumps heat Q2, and then returns to its original, unaltered state.
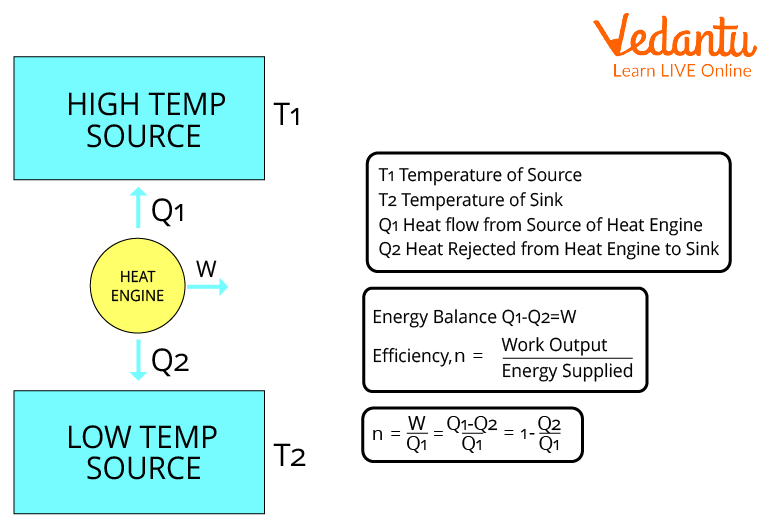
Heat Engine and Efficiency
Consider the universe's net change in entropy \[\Delta S\]\[\].
At a steady temperature T1, the heat reservoir emits heat Q1. As a result, the change in its entropy is:
\[\Delta {S_1} = - \frac{{{Q_1}}}{{{T_1}}}\]
At a steady temperature T2, the heat sink receives heat Q2. As a result, the change in its entropy is:
\[\Delta {S_2} = - \frac{{{Q_2}}}{{{T_2}}}\]
As a result, the universe's net entropy change is,
\[\begin{array}{l}\Delta S = \Delta {S_1} + \Delta {S_2}\\\Delta S = \frac{{{Q_2}}}{{{T_2}}} - \frac{{{Q_1}}}{{{T_1}}}\end{array}\]
Using the second law of thermodynamics, \[\Delta S \ge 0\], it follows that
\[\begin{array}{l}\frac{{{Q_2}}}{{{T_2}}} - \frac{{{Q_1}}}{{{T_1}}} \ge 0\\\frac{{{Q_2}}}{{{T_2}}} \ge \frac{{{Q_1}}}{{{T_1}}}\\\frac{{{Q_2}}}{{{Q_1}}} - \frac{{{T_2}}}{{{T_1}}}\\1 - \frac{{{Q_2}}}{{{Q_1}}} \le 1 - \frac{{{T_2}}}{{{T_1}}}\end{array}\]
Because the left side reflects the efficiency of the supplied heat engine \[\eta\] and the right side indicates the efficiency of a Carnot engine,
\[\eta \le {\eta _{carnot}} \to {\eta _{\max }} = {\eta _{carnot}}\]
Hence proved
Limitations of Carnot Theorem
The Carnot Cycle is an ideal cycle, which means it does not exist and cannot be created. Hence it is only a theoretical concept.
The Carnot Cycle is only used to examine heat engines and does not apply to other sorts of devices.
The output obtained per cycle is quite low.
Carnot Cycle Application
Carnot's theorem is used in engines that turn thermal energy into work.
In a refrigeration system, the Carnot cycle is reversed. We know that a heat engine based on the Carnot engine has the maximum efficiency. Similarly, a change cycle refrigeration system has the highest coefficient of performance.
Solved Examples
1. What is the efficiency of a Carnot engine operating between \[{340^ \circ }C\] and \[{70^ \circ }C\]?
Ans. Given data:
\[\begin{array}{l}{T_1} = {340^ \circ }C = (340 + 273)K\\{T_1} = 613K\\{T_2} = {70^ \circ }C = (70 + 273)K\\{T_2} = 343K\end{array}\]
Carnot's engine efficiency is given by,
\[\begin{array}{l}\eta = 1 - (\frac{{{T_2}}}{{{T_1}}})\\\therefore \,\eta = 1 - (\frac{{343}}{{613}})\\ \Rightarrow \frac{{613 - 343}}{{613}} \approx 0.44\end{array}\]
Therefore, the efficiency of the Carnot engine operating between the given temperatures is about \[44\].
2. A heat engine with a 55% efficiency performs 70kJ of work every Carnot cycle. What amount of heat is rejected in each cycle?
Ans. Given data:
\[\begin{array}{l}\eta = 55\% \\ \Rightarrow \,\eta = 0.55\\w = 70J\end{array}\]
As known,
\[\eta \] = output work/Heat absorbed \[{Q_1}\]
Therefore,
\[\begin{array}{l}0.55 = \frac{{70}}{{{Q_1}}}\\ \Rightarrow {Q_1} = 127.27\end{array}\]
Work = \[{Q_1} - {Q_2}\] (Heat absorbed - Heat rejected)
\[\begin{array}{l}70 = {Q_1} - 127.27\\{Q_1} = 57.27kJ\end{array}\]
So, the amount of heat rejected in each cycle is \[57.27kJ\].
3. Consider Carnot's cycle that produces \[2kJ\] of mechanical work during each cycle and operates between \[{T_1} = 400K\] and \[{T_2} = 560K\]. What is the amount of heat transmitted to the engine by the reservoirs?
Ans. Given data:
\[{T_1} = 400K\]
\[{T_2} = 560K\]
Output Work = \[2kJ\]
Carnot's engine efficiency is given by,
\[\begin{array}{l}\eta = 1 - \frac{{{T_2}}}{{{T_1}}}\\ \Rightarrow \eta = 1 - \frac{{400}}{{560}}\\ \Rightarrow \eta = \frac{{560 - 400}}{{560}}\\ \Rightarrow \eta = 0.28\end{array}\]
Efficiency can also be written as:
\[\eta = \dfrac {\text{output~ work}}{\text{Heat~ supplied}}\]
\[\eta = \dfrac{{2000}}{x}\]
Where ‘x’ is the total heat supplied.
Now substitute the calculated value of efficiency in the above expression.
\[\begin{array}{l}0.28 = \frac{{2000}}{x}\\ \Rightarrow 0.28x = 2000\\ \Rightarrow x = \frac{{2000}}{{0.28}}\\ \Rightarrow x = 7142.85J\end{array}\]
Important Points to Remember
A Carnot cycle employs an ideal gas as a working fluid.
The working substance is subjected to a cyclic operation involving two isothermal and two adiabatic operations in a Carnot cycle.
Important Formulas to Remember from Theorem
The formula for maximum efficiency is as follows:
\[\eta {}_{\max } = 1 - \frac{{{T_2}}}{{{T_1}}}\]
Where \[\eta \] is the efficiency, \[{T_2}\] is the absolute temperature (Kelvins) of the cold reservoir, and \[{T_1}\] is the absolute temperature (Kelvins) of the hot reservoir.
Another formula for efficiency,
\[\eta \] = $\dfrac {\text{output~ work}}{\text{Heat~ supplied}}$
Conclusion
Carnot's theorem illustrates the principle that an irreversible engine's efficiency cannot be greater than that of a reversible engine if both operate between fixed temperature heat reservoirs.
Simple heat engines are less effective and efficient when employed between two heat reservoirs, but Carnot heat engines are more efficient. Regardless of their operation, the Carnot engines are always efficient.
FAQs on Carnot Theorem Explained: Principle, Proof & Applications
1. What is the fundamental principle of Carnot's theorem?
Carnot's theorem states that no heat engine operating between two specific heat reservoirs can be more efficient than a reversible Carnot engine operating between the same two reservoirs. It essentially establishes the maximum theoretical efficiency for any heat engine, which depends only on the temperatures of the hot and cold reservoirs.
2. What are the four main processes that make up a Carnot cycle?
A Carnot cycle is an ideal, reversible thermodynamic cycle composed of four distinct processes:
- Isothermal Expansion: The working substance absorbs heat from a high-temperature reservoir (T_H) and expands at a constant temperature.
- Adiabatic Expansion: The substance continues to expand without any heat exchange, causing its temperature to decrease to that of the cold reservoir (T_C).
- Isothermal Compression: The substance releases heat to the cold reservoir (T_C) as it is compressed at a constant temperature.
- Adiabatic Compression: The substance is further compressed without heat exchange, raising its temperature back to T_H and returning it to its initial state.
3. What is the formula for calculating the efficiency of a Carnot engine?
The efficiency (η) of a Carnot engine is given by the formula: η_carnot = 1 - (T_C / T_H). In this equation, T_C is the absolute temperature (in Kelvin) of the cold reservoir (sink), and T_H is the absolute temperature (in Kelvin) of the hot reservoir (source). This formula defines the maximum possible efficiency.
4. What are the practical implications and applications of Carnot's theorem?
While the Carnot engine itself is a theoretical ideal, its theorem is crucial for practical engineering. It serves as a fundamental benchmark to:
- Evaluate and compare the performance of real-world heat engines, such as those in power plants and automobiles.
- Guide engineers in optimising the design of refrigerators and heat pumps.
- Understand the ultimate physical limits on converting thermal energy into useful mechanical work.
5. Why is a real-world engine always less efficient than a Carnot engine?
A real-world engine's efficiency is always lower than a Carnot engine's because real processes are irreversible. The Carnot cycle assumes perfect, frictionless, and infinitely slow processes that are fully reversible. In reality, factors like friction between moving parts, heat loss to the surroundings, and the finite speed of operations introduce irreversibilities, which dissipate energy and reduce the overall efficiency.
6. How is Carnot's theorem a consequence of the Second Law of Thermodynamics?
Carnot's theorem is fundamentally rooted in the Second Law of Thermodynamics. The second law states that the total entropy (disorder) of an isolated system can never decrease. If a heat engine existed that was more efficient than a Carnot engine, it could be used in conjunction with a reversed Carnot engine to create a machine that transfers heat from a cold body to a hot body without any external work. This would result in a net decrease in entropy, which directly violates the second law.
7. What are the primary limitations of the Carnot cycle in practical applications?
The Carnot cycle is not used for practical engines due to several major limitations. The isothermal and adiabatic processes must be carried out extremely slowly to maintain thermal equilibrium and be reversible. This would lead to an engine with a very low power output. Additionally, creating a real machine with perfect insulation for the adiabatic steps and a frictionless mechanism is technologically impossible. Therefore, it remains a vital theoretical model rather than a practical blueprint.
























