




Key Examples and Properties of Poor Conductors
Why is it said not to plug a metal object like a fork into an electric socket? That is because you can get an electric shock, but how and why is that possible? Have you ever wondered why some materials can pass on an electric current to the next object while other materials can’t?
Materials that can easily conduct electricity or material that allow electricity to easily pass through them are known as conductors. Metal is an example of a good conductor. Since metal allows electricity to pass through it easily, when a metal object is plugged into an electric socket, the electricity passes through it, and the person holding the object receives an electrical shock. Wood is one of the poorest conductors of electricity.
Conductors and Insulators
Conductors vs Insulators
Conductors allow electricity to easily pass through them. Substances known as insulators are materials that do not allow electricity to pass through them easily or at all.
Conductors - Metals, water, humans
Insulators - Rubber, wood, plastic
Conductors
How does a conductor allow electricity to flow through it? In materials such as metals, which make one of the best conductors, there is an easy flow of ions and electrons once a voltage is applied. This easy flow of ions from atom to atom allows the metal to conduct electricity.
Conductors have a very low resistance to electric current.
Insulators or Bad Conductors
Atoms in materials such as rubber have tightly bound electrons, which prevent the flow of electricity. Insulators or Bad/Poor conductors, thus, are materials that do not allow free and easy flow of ions and electrons from atom to atom. Most non-metals make bad conductors as this easy flow of ions is not possible. Poor Conductors of electricity have a high resistance to electric current.
Have you ever seen electric wires? These wires are made of metals such as copper, which are great conductors to allow an electric current to flow. But these copper wires are often covered with rubber which is a poor conductor of electricity to prevent anyone from getting an electric shock when they come in contact with the wire.
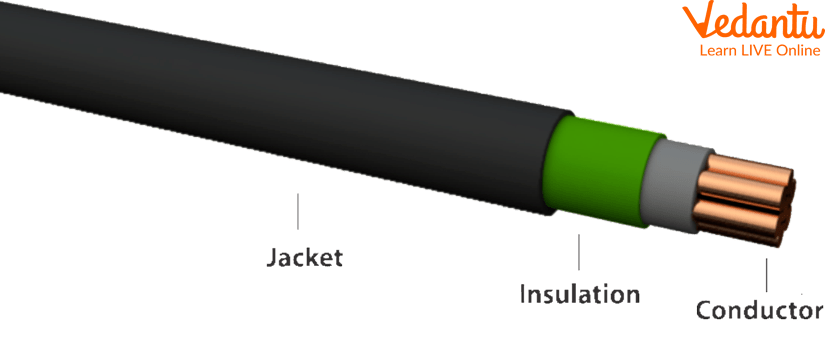
Rubber Insulated Wires
Why are Covalent Compounds Generally Poor Conductors of Electricity?
Covalent compounds are molecules that are made up of more than one atom pair sharing valence electrons. These compounds have covalent bonds. Examples of such compounds are Oxygen, Nitrogen, etc.
These covalent compounds are bad conductors of electricity as these molecules are made by atoms sharing electrons. Thus, there are no free electrons left to conduct electricity.
Interesting Facts about Insulators
Covalent compounds are bad conductors of electricity.
High temperatures can make good conductors not conduct electricity well.
Dielectrics are insulating materials, which when placed in an electric circuit, have a polarising effect (positive charges move toward the electric field and negative charges move in the opposite direction of the electric field).
Substances that are bad conductors of heat are often bad conductors of electricity also.
Summary
Insulators or bad conductors are materials that do not allow electricity to easily pass through them. Atoms in such material have tightly bound electrons, which prevent the flow of electric current. Examples of bad conductors include - Wood, Rubber, Glass, Sand, etc.
FAQs on Poor Conductors of Electricity Explained
1. What are poor conductors of electricity?
Materials that do not allow electricity to pass through them easily are called poor conductors of electricity. They are also commonly known as insulators. These materials effectively block or resist the flow of an electric current, making them essential for electrical safety.
2. What are some common examples of poor conductors of electricity found at home?
Many everyday items are poor conductors of electricity. Common examples include:
- Plastic (used in toys, switches, and wire coverings)
- Rubber (used in gloves and shoe soles)
- Wood (like a dry wooden spoon or furniture)
- Glass (found in windows and bulbs)
- Dry cloth and paper
3. What is the main difference between a good conductor and a poor conductor of electricity?
The main difference lies in how easily they allow electricity to flow. A good conductor, such as a copper wire or an iron nail, lets electric current pass through it with very little resistance. In contrast, a poor conductor, like a plastic ruler or a rubber eraser, has very high resistance and blocks the flow of electricity.
4. Why are electrical wires always covered with a layer of plastic or rubber?
Electrical wires are covered with plastic or rubber for safety. The metal inside the wire is a good conductor that carries the electric current. The plastic or rubber coating acts as an insulator (a poor conductor), which prevents the electricity from flowing to the outside. This protective layer stops us from getting a dangerous electric shock if we accidentally touch the wire.
5. Why are materials like wood and plastic considered poor conductors?
Materials such as wood and plastic are poor conductors because of their internal structure. For electricity to flow, tiny charged particles called electrons must be able to move freely. In insulators like wood and plastic, these electrons are held very tightly to their atoms and cannot move easily. Since there is no free movement of charge, the electric current is blocked.
6. Why is it extremely dangerous to touch electrical switches or appliances with wet hands?
This is a critical safety rule because while pure water is a poor conductor, the water we use daily (tap water) contains dissolved salts and minerals. These impurities make water a good conductor of electricity. Touching an electrical device with wet hands creates a path for the current to flow from the device, through the water, and into your body, which can cause a severe and potentially fatal electric shock.
7. Are materials that are poor conductors of electricity also poor conductors of heat?
Yes, in many cases, materials that are poor electrical conductors are also poor heat conductors. For example, a plastic handle on a cooking pot does not get hot quickly because plastic is a poor conductor of heat. It is also a poor conductor of electricity. This is why materials like wood, plastic, and rubber are used for both electrical insulation and heat insulation.
8. Can a poor conductor, like air, ever conduct electricity?
Yes, under certain conditions. While air is normally an excellent insulator (poor conductor), a very strong electric field can force electricity to pass through it. A powerful example of this is lightning. During a thunderstorm, a massive electrical charge builds up, becoming strong enough to break down the air's resistance and travel through it as a lightning strike.









