
Zinc Sulphate forms a colourless solution in water. Colour on adding copper is:
(a) Blue
(b) Black
(c) Brown
(d) No change in colour
Answer
609.6k+ views
Hint: One needs to understand the relative position of the elements in the metal reactivity series. The particular snippet of the same is given in the table form here below:
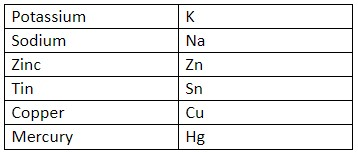
In this snippet of the series, Potassium is the most reactive and the reactivity goes on decreasing as we go down.
Complete Step by Step Solution:
All in all, it is very important for one to understand the concept of metal reactivity series. Through that understanding, it is very much possible for one to answer this problem.
So, the metal reactivity series is a series of metal which is used to determine the product of single displacement reactions, whereby a metal A will displace another metal, say C, in a solution if A is higher in the series than C.
Now we know that the colour of copper is cobalt-blue, but while referring to the problem in which the Zinc sulphate is undergoing a displacement reaction with Copper. There won’t be any reaction that would be taking place. Why? Because as we see Copper is placed below Zinc in the metal reactivity series and hence there won’t be any reaction that would be taking place.
This also shows the fact to us that Copper is not a strong metal to react with zinc sulphate solution and therefore cannot replace from its respective salt.
Hence, the answer is D which is that there won’t be any colour change.
Note: Well, if the solution of Zinc sulphate in the concerned question was to react with Potassium or Sodium for that matter, there would be a displacement reaction that would take place and there would be respective colour changes as per the reaction.

In this snippet of the series, Potassium is the most reactive and the reactivity goes on decreasing as we go down.
Complete Step by Step Solution:
All in all, it is very important for one to understand the concept of metal reactivity series. Through that understanding, it is very much possible for one to answer this problem.
So, the metal reactivity series is a series of metal which is used to determine the product of single displacement reactions, whereby a metal A will displace another metal, say C, in a solution if A is higher in the series than C.
Now we know that the colour of copper is cobalt-blue, but while referring to the problem in which the Zinc sulphate is undergoing a displacement reaction with Copper. There won’t be any reaction that would be taking place. Why? Because as we see Copper is placed below Zinc in the metal reactivity series and hence there won’t be any reaction that would be taking place.
This also shows the fact to us that Copper is not a strong metal to react with zinc sulphate solution and therefore cannot replace from its respective salt.
Hence, the answer is D which is that there won’t be any colour change.
Note: Well, if the solution of Zinc sulphate in the concerned question was to react with Potassium or Sodium for that matter, there would be a displacement reaction that would take place and there would be respective colour changes as per the reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




