
Zinc sulphate crystals are hydrated.They contain water of crystallization.A student did an experiment to find the mass of water in the hydrated crystals.The crystals were weighed and then heated with a Bunsen burner.
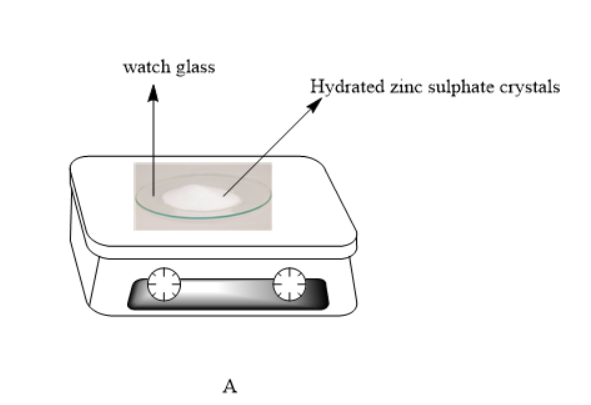
1)Name the apparatus used to weigh the crystal in A
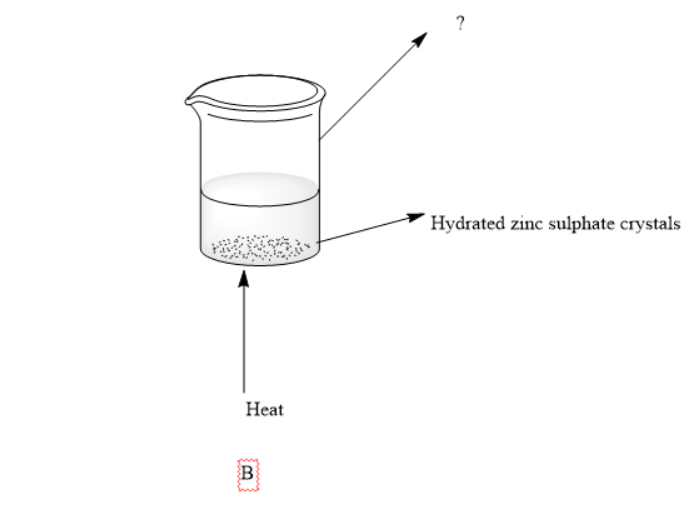
2)Complete the box in apparatus B
3)What should be the position of the hole of Bunsen burner in apparatus B


Answer
498.3k+ views
Hint: When compounds undergo hydration they experience an increase in weight.By weighing them we can estimate the water content in them.It is also worth noting that hydration of compounds is reversible for most of the compounds and this can be done as easily by hearing the hydrated compound.In the above given experiment also the student is trying to do something similar.
Complete answer:
Zinc sulphate is an organic compound.Its often used for making zinc dietary supplements.It was also known as white vitriol.The chemical formula for zinc sulphate is $ZnS{O_4}$ .However one of its most common form is in the form of waters of crystallisation which has a chemical formula of $ZnS{O_4}.7{H_2}O$ .
In the above experiment the student started out with non hydrated Zinc sulphate crystals.He then went to hydrate them.After hydration the weight of the crystals increased.The student got very curious and wanted to know the mass of water in the hydrated zinc sulphate crystals.
Therefore he collected the hydrated crystals in a watch glass and placed the watch glass on an electronic balance.This helped him in getting an accurate measurement of weight of the hydrated crystals.
After this he decided to dehydrate the crystals for this he put the hydrated crystals in a beaker and heated them using a Bunsen burner.The hole of the Bunsen burner is kept open.
Note:
For weighing the hydrated crystals an electronic balance is used since it gives accurate measurements.Bunsen burner is used for heating compounds in laboratories.It has a small adjustable air nob at the bottom which helps in adjusting the type of flame.
Complete answer:
Zinc sulphate is an organic compound.Its often used for making zinc dietary supplements.It was also known as white vitriol.The chemical formula for zinc sulphate is $ZnS{O_4}$ .However one of its most common form is in the form of waters of crystallisation which has a chemical formula of $ZnS{O_4}.7{H_2}O$ .
In the above experiment the student started out with non hydrated Zinc sulphate crystals.He then went to hydrate them.After hydration the weight of the crystals increased.The student got very curious and wanted to know the mass of water in the hydrated zinc sulphate crystals.
Therefore he collected the hydrated crystals in a watch glass and placed the watch glass on an electronic balance.This helped him in getting an accurate measurement of weight of the hydrated crystals.
After this he decided to dehydrate the crystals for this he put the hydrated crystals in a beaker and heated them using a Bunsen burner.The hole of the Bunsen burner is kept open.
Note:
For weighing the hydrated crystals an electronic balance is used since it gives accurate measurements.Bunsen burner is used for heating compounds in laboratories.It has a small adjustable air nob at the bottom which helps in adjusting the type of flame.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




